The Arabidopsis homolog of trithorax, ATX1, binds phosphatidylinositol 5-phosphate, and the two regulate a common set of target genes
- PMID: 16585509
- PMCID: PMC1458695
- DOI: 10.1073/pnas.0600944103
The Arabidopsis homolog of trithorax, ATX1, binds phosphatidylinositol 5-phosphate, and the two regulate a common set of target genes
Abstract
The Arabidopsis homolog of trithorax, ATX1, regulates numerous functions in Arabidopsis beyond the homeotic genes. Here, we identified genome-wide targets of ATX1 and showed that ATX1 is a receptor for a lipid messenger, phosphatidylinositol 5-phosphate, PI5P. PI5P negatively affects ATX1 activity, suggesting a regulatory pathway connecting lipid-signaling with nuclear functions. We propose a model to illustrate how plants may respond to stimuli (external or internal) that elevate cellular PI5P levels by altering expression of ATX1-controlled genes.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
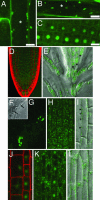


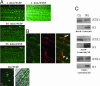

References
-
- Simon J. A., Tamkun J. W. Curr. Opin. Genet. Dev. 2002;12:210–218. - PubMed
-
- Schwartz-Sommer Z., Huijser P., Nacken W., Saedler H., Sommer H. Science. 1990;250:931–936. - PubMed
-
- Alvarez-Venegas R., Pien S., Sadder M., Witmer X., Grossniklaus U., Avramova Z. Curr. Biol. 2003;13:627–637. - PubMed
-
- Rea S., Eisenhaber F., O’Carroll D., Strahl B. D., Sun Z.-W., Schmid M., Opravil S., Mechtler K., Pontig C. P., Allis C. D., Jenuwein T. Nature. 2000;406:593–599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

