Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival
- PMID: 16585512
- PMCID: PMC1458653
- DOI: 10.1073/pnas.0510923103
Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival
Abstract
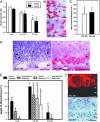
Malignant gliomas remain incurable brain tumors because of their diffuse-invasive growth. So far, the genetic and molecular events underlying gliomagenesis are poorly understood. In this study, we have identified the receptor tyrosine kinase Axl as a mediator of glioma growth and invasion. We demonstrate that Axl and its ligand Gas6 are overexpressed in human glioma cell lines and that Axl is activated under baseline conditions. Furthermore, Axl is expressed at high levels in human malignant glioma. Inhibition of Axl signaling by overexpression of a dominant-negative receptor mutant (AXL-DN) suppressed experimental gliomagenesis (growth inhibition >85%, P < 0.05) and resulted in long-term survival of mice after intracerebral glioma cell implantation when compared with Axl wild-type (AXL-WT) transfected tumor cells (survival times: AXL-WT, 10 days; AXL-DN, >72 days). A detailed analysis of the distinct hallmarks of glioma pathology, such as cell proliferation, migration, and invasion and tumor angiogenesis, revealed that inhibition of Axl signaling interfered with cell proliferation (inhibition 30% versus AXL-WT), glioma cell migration (inhibition 90% versus mock and AXL-WT, P < 0.05), and invasion (inhibition 62% and 79% versus mock and AXL-WT, respectively; P < 0.05). This study describes the identification, functional manipulation, in vitro and in vivo validation, and preclinical therapeutic inhibition of a target receptor tyrosine kinase mediating glioma growth and invasion. Our findings implicate Axl in gliomagenesis and validate it as a promising target for the development of approaches toward a therapy of these highly aggressive but, as yet, therapy-refractory, tumors.
Conflict of interest statement
Conflict of interest statement: P.V., P.K., and A.U. have filed a patent on the role of Axl in tumor biology. The patent has been licensed by the company U3, which pursues Axl as a potential target for antitumor therapy.
Figures




References
-
- DeAngelis L. M. N. Engl. J. Med. 2001;344:114–123. - PubMed
-
- Stupp R., Mason W. P., van den Bent M. J., Weller M., Fisher B., Taphoorn M. J., Belanger K., Brandes A. A., Marosi C., Bogdahn U., et al. N. Engl. J. Med. 2005;352:987–996. - PubMed
-
- Lefranc F., Brotchi J., Kiss R. J. Clin. Oncol. 2005;23:2411–2422. - PubMed
-
- Ullrich A., Schlessinger J. Cell. 1990;61:203–212. - PubMed
-
- Janssen J. W., Schulz A. S., Steenvoorden A. C., Schmidberger M., Strehl S., Ambros P. F., Bartram C. R. Oncogene. 1991;6:2113–2120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

