Overlapping hand-over-hand mechanism of single molecular motility of cytoplasmic dynein
- PMID: 16585530
- PMCID: PMC1424663
- DOI: 10.1073/pnas.0508511103
Overlapping hand-over-hand mechanism of single molecular motility of cytoplasmic dynein
Abstract
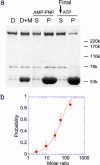
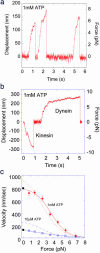
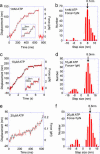
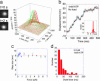
Structural differences between dynein and kinesin suggest a unique molecular mechanism of dynein motility. Measuring the mechanical properties of a single molecule of dynein is crucial for revealing the mechanisms underlying its movement. We measured the step size and force produced by single molecules of active cytoplasmic dynein by using an optical trap and fluorescence imaging with a high temporal resolution. The velocity of dynein movement, 800 nm/s, is consistent with that reported in cells. The maximum force of 7-8 pN was independent of the ATP concentration and similar to that of kinesin. Dynein exhibited forward and occasional backwards steps of approximately 8 nm, independent of load. It is suggested that the large dynein heads take 16-nm steps by using an overlapping hand-over-hand mechanism.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

