Transcriptional heat shock response in the smallest known self-replicating cell, Mycoplasma genitalium
- PMID: 16585746
- PMCID: PMC1447023
- DOI: 10.1128/JB.188.8.2845-2855.2006
Transcriptional heat shock response in the smallest known self-replicating cell, Mycoplasma genitalium
Abstract
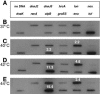
Mycoplasma genitalium is a human bacterial pathogen linked to urethritis and other sexually transmitted diseases as well as respiratory and joint pathologies. Though its complete genome sequence is available, little is understood about the regulation of gene expression in this smallest known, self-replicating cell, as its genome lacks orthologues for most of the conventional bacterial regulators. Still, the transcriptional repressor HrcA (heat regulation at CIRCE [controlling inverted repeat of chaperone expression]) is predicted in the M. genitalium genome as well as three copies of its corresponding regulatory sequence CIRCE. We investigated the transcriptional response of M. genitalium to elevated temperatures and detected the differential induction of four hsp genes. Three of the up-regulated genes, which encode DnaK, ClpB, and Lon, possess CIRCE within their promoter regions, suggesting that the HrcA-CIRCE regulatory mechanism is functional. Additionally, one of three DnaJ-encoding genes was up-regulated, even though no known regulatory sequences were found in the promoter region. Transcript levels returned to control values after 1 h of incubation at 37 degrees C, reinforcing the transient nature of the heat shock transcriptional response. Interestingly, neither of the groESL operon genes, which encode the GroEL chaperone and its cochaperone GroES, responded to heat shock. These data suggest that M. genitalium selectively regulates a limited number of genes in response to heat shock.
Figures




References
-
- Bang, H., A. Pecht, G. Raddatz, T. Scior, W. Solbach, K. Brune, and A. Pahl. 2000. Prolyl isomerases in a minimal cell. Catalysis of protein folding by trigger factor from Mycoplasma genitalium. Eur. J. Biochem. 267:3270-3280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

