Ontogeny of adrenal steroid biosynthesis: why girls will be girls
- PMID: 16585958
- PMCID: PMC1421368
- DOI: 10.1172/JCI28296
Ontogeny of adrenal steroid biosynthesis: why girls will be girls
Abstract
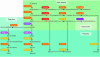
Male and female external genitalia appear identical early in gestation. Testosterone exposure at 8-12 weeks' gestation causes male differentiation. Female fetuses virilize if their adrenals secrete excessive levels of androgens, as occurs in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. This can be ameliorated by administering dexamethasone to the mother. A study by Goto et al. in this issue of the JCI provides a rationale for this treatment by demonstrating that the fetal hypothalamic-pituitary-adrenal axis is fully functional when the genitalia differentiate (see the related article beginning on page 953). Dexamethasone suppresses this axis, reducing abnormal secretion of adrenal androgens. Their results also show that cortisol synthesis by the fetal adrenal decreases after this period, allowing the adrenal to secrete high levels of dehydroepiandrosterone, an androgen precursor. However, this does not virilize female fetuses because androgens are aromatized to estrogens in the placenta. Thus normal sexual differentiation requires exquisite timing of fetal cortisol and androgen secretion versus placental capacity for aromatization.
Figures

Comment on
-
In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development.J Clin Invest. 2006 Apr;116(4):953-60. doi: 10.1172/JCI25091. J Clin Invest. 2006. PMID: 16585961 Free PMC article.
References
-
- Goto M., et al. Steroidogenic enzyme expression within the adrenal cortex during early human gestation. Endocr. Res. 2002;28:641–645. - PubMed
-
- Simpson E.R., MacDonald P.C. Endocrine physiology of the placenta. Annu. Rev. Physiol. 1981;43:163–188. - PubMed
-
- Mendelson C.R., Condon J.C. New insights into the molecular endocrinology of parturition. J. Steroid Biochem. Mol. Biol. 2005;93:113–119. - PubMed
-
- White P.C., Mune T., Agarwal A.K. 11β-hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr. Rev. 1997;18:135–156. - PubMed

