In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development
- PMID: 16585961
- PMCID: PMC1421344
- DOI: 10.1172/JCI25091
In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development
Abstract
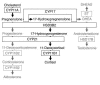
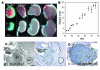
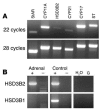
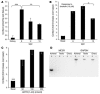
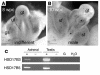
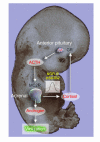
In humans, sexual differentiation of the external genitalia is established at 7-12 weeks post conception (wpc). During this period, maintaining the appropriate intrauterine hormone environment is critical. In contrast to other species, this regulation extends to the human fetal adrenal cortex, as evidenced by the virilization that is associated with various forms of congenital adrenal hyperplasia. The mechanism underlying these clinical findings has remained elusive. Here we show that the human fetal adrenal cortex synthesized cortisol much earlier than previously documented, an effect associated with transient expression of the orphan nuclear receptor nerve growth factor IB-like (NGFI-B) and its regulatory target, the steroidogenic enzyme type 2 3beta-hydroxysteroid dehydrogenase (HSD3B2). This cortisol biosynthesis was maximal at 8-9 wpc under the regulation of ACTH. Negative feedback was apparent at the anterior pituitary corticotrophs. ACTH also stimulated the adrenal gland to secrete androstenedione and testosterone. In concert, these data promote a distinctive mechanism for normal human development whereby cortisol production, determined by transient NGFI-B and HSD3B2 expression, provides feedback at the anterior pituitary to modulate androgen biosynthesis and safeguard normal female sexual differentiation.
Figures


Comment in
-
Ontogeny of adrenal steroid biosynthesis: why girls will be girls.J Clin Invest. 2006 Apr;116(4):872-4. doi: 10.1172/JCI28296. J Clin Invest. 2006. PMID: 16585958 Free PMC article.
References
-
- White P.C., Speiser P.W. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr. Rev. 2000;21:245–291. - PubMed
-
- David M., Forest M.G. Prenatal treatment of congenital adrenal hyperplasia resulting from 21-hydroxylase deficiency. J. Pediatr. . 1984;105:799–803. - PubMed
-
- Mesiano S., Jaffe R.B. Developmental and functional biology of the primate fetal adrenal cortex. Endocr. Rev. 1997;18:378–403. - PubMed
-
- Pepe G.J., Albrecht E.D. Regulation of the primate fetal adrenal cortex. Endocr. Rev. 1990;11:151–176. - PubMed
-
- Narasaka T., Suzuki T., Moriya T., Sasano H. Temporal and spatial distribution of corticosteroidogenic enzymes immunoreactivity in developing human adrenal. Mol. Cell. Endocrinol. . 2001;174:111–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

