Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis
- PMID: 16585964
- PMCID: PMC1421348
- DOI: 10.1172/JCI25790
Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis
Abstract
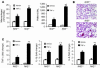
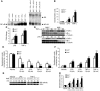
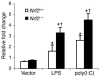
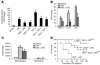
Host genetic factors that regulate innate immunity determine susceptibility to sepsis. Disruption of nuclear factor-erythroid 2-related factor 2 (Nrf2), a basic leucine zipper transcription factor that regulates redox balance and stress response, dramatically increased the mortality of mice in response to endotoxin- and cecal ligation and puncture-induced septic shock. LPS as well as TNF-alpha stimulus resulted in greater lung inflammation in Nrf2-deficient mice. Temporal analysis of pulmonary global gene expression after LPS challenge revealed augmented expression of large numbers of proinflammatory genes associated with the innate immune response at as early as 30 minutes in lungs of Nrf2-deficient mice, indicating severe immune dysregulation. The expression profile indicated that Nrf2 has a global influence on both MyD88-dependent and -independent signaling. Nrf2-deficient mouse embryonic fibroblasts showed greater activation of NF-kappaB and interferon regulatory factor 3 in response to LPS and polyinosinic-polycytidylic acid [poly(I:C)] stimulus, corroborating the effect of Nrf2 on MyD88-dependent and -independent signaling. Nrf2's regulation of cellular glutathione and other antioxidants is critical for optimal NF-kappaB activation in response to LPS and TNF-alpha. Our study reveals Nrf2 as a novel modifier gene of sepsis that determines survival by mounting an appropriate innate immune response.
Figures
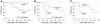
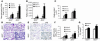
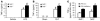

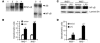


Comment in
-
Oxidative stress in sepsis: a redox redux.J Clin Invest. 2006 Apr;116(4):860-3. doi: 10.1172/JCI28111. J Clin Invest. 2006. PMID: 16585954 Free PMC article.
References
-
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. - PubMed
-
- Pinsky M.R. Dysregulation of the immune response in severe sepsis. Am. J. Med. Sci. 2004;328:220–229. - PubMed
-
- Angus D.C., et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. - PubMed
-
- Arcaroli J., Fessler M.B., Abraham E. Genetic polymorphisms and sepsis. Shock. 2005;24:300–312. - PubMed
-
- Thimmulappa R.K., et al. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

