Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model
- PMID: 16595634
- PMCID: PMC1458691
- DOI: 10.1073/pnas.0509227103
Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model
Abstract
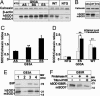
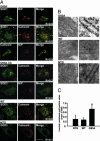
Mutation in superoxide dismutase-1 (SOD1), which is a cause of ALS, alters the folding patterns of this protein. Accumulation of misfolded mutant SOD1 might activate endoplasmic reticulum (ER) stress pathways. Here we show that transgenic mice expressing ALS-linked SOD1 mutants exhibit molecular alterations indicative of a recruitment of ER's signaling machinery. We demonstrate by biochemical and morphological methods that mutant SOD1 accumulates inside the ER, where it forms insoluble high molecular weight species and interacts with the ER chaperone immunoglobulin-binding protein. These alterations are age- and region-specific, because they develop over the course of the disease and occur in the affected spinal cord but not in the nonaffected cerebellum in transgenic mutant SOD1 mice. Our results suggest a toxic mechanism for mutant SOD1 by which this ubiquitously expressed pathogenic protein could affect motor neuron survival and contribute to the selective motor neuronal degeneration in ALS.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

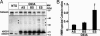

References
-
- Julien J. P. Cell. 2001;104:581–591. - PubMed
-
- Bruijn L. I., Houseweart M. K., Kato S., Anderson K. L., Anderson S. D., Ohama E., Reaume A. G., Scott R. W., Cleveland D. W. Science. 1998;281:1851–1854. - PubMed
-
- Bruijn L. I., Becher M. W., Lee M. K., Anderson K. L., Jenkins N. A., Copeland N. G., Sisodia S., Rothstein J. D., Borchelt D. R., Price D. L., et al. Neuron. 1997;18:327–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

