Local osmosis and isotonic transport
- PMID: 16596445
- PMCID: PMC1752196
- DOI: 10.1007/s00232-005-0817-9
Local osmosis and isotonic transport
Abstract
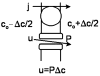
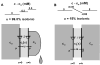
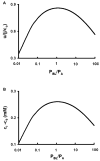
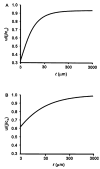
Osmotically driven water flow, u (cm/s), between two solutions of identical osmolarity, c(o) (300 mM: in mammals), has a theoretical isotonic maximum given by u = j/c(o), where j (moles/cm(2)/s) is the rate of salt transport. In many experimental studies, transport was found to be indistinguishable from isotonic. The purpose of this work is to investigate the conditions for u to approach isotonic. A necessary condition is that the membrane salt/water permeability ratio, epsilon, must be small: typical physiological values are epsilon = 10(-3) to 10(-5), so epsilon is generally small but this is not sufficient to guarantee near-isotonic transport. If we consider the simplest model of two series membranes, which secrete a tear or drop of sweat (i.e., there are no externally-imposed boundary conditions on the secretion), diffusion is negligible and the predicted osmolarities are: basal = c(o), intracellular approximately (1 + epsilon)c(o), secretion approximately (1 + 2epsilon)c(o), and u approximately (1 - 2epsilon)j/c(o). Note that this model is also appropriate when the transported solution is experimentally collected. Thus, in the absence of external boundary conditions, transport is experimentally indistinguishable from isotonic. However, if external boundary conditions set salt concentrations to c(o) on both sides of the epithelium, then fluid transport depends on distributed osmotic gradients in lateral spaces. If lateral spaces are too short and wide, diffusion dominates convection, reduces osmotic gradients and fluid flow is significantly less than isotonic. Moreover, because apical and basolateral membrane water fluxes are linked by the intracellular osmolarity, water flow is maximum when the total water permeability of basolateral membranes equals that of apical membranes. In the context of the renal proximal tubule, data suggest it is transporting at near optimal conditions. Nevertheless, typical physiological values suggest the newly filtered fluid is reabsorbed at a rate u approximately 0.86 j/c(o), so a hypertonic solution is being reabsorbed. The osmolarity of the filtrate c(F) (M) will therefore diminish with distance from the site of filtration (the glomerulus) until the solution being transported is isotonic with the filtrate, u = j/c(F).With this steady-state condition, the distributed model becomes approximately equivalent to two membranes in series. The osmolarities are now: c(F) approximately (1 - 2epsilon)j/c(o), intracellular approximately (1 - epsilon)c(o), lateral spaces approximately c(o), and u approximately (1 + 2epsilon)j/c(o). The change in c(F) is predicted to occur with a length constant of about 0.3 cm. Thus, membrane transport tends to adjust transmembrane osmotic gradients toward epsilonc(o), which induces water flow that is isotonic to within order epsilon. These findings provide a plausible hypothesis on how the proximal tubule or other epithelia appear to transport an isotonic solution.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

