De novo asymmetric synthesis of D- and L-swainsonine
- PMID: 16597122
- PMCID: PMC2531217
- DOI: 10.1021/ol0602811
De novo asymmetric synthesis of D- and L-swainsonine
Abstract
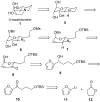
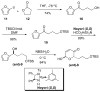
[reaction: see text] The enantioselective syntheses of both enantiomers of the indolizidine natural product swainsonine have been achieved in 13 steps from furan. The indolizidine ring system is installed by a one-pot hydrogenolysis of both an azide and an O-Bn group along with an intramolecular reductive amination reaction. The asymmetry of swainsonine was introduced by Noyori reduction of an acylfuran. This route relies upon an Achmatowicz rearrangement, a diastereoselective palladium-catalyzed glycosylation, Luche reduction, and a dihydroxylation reaction.
Figures





References
-
- Asano N, Nash RJ, Molyneux RJ, Fleet GWJ. Tetrahedron: Asymmetry. 2000;11:1645–1680.
- Michael JP. Nat Prod Rep. 1997;14:619–636. - PubMed
-
-
For a review of iminosugars: Sears P, Wong CH. Angew Chem, Int Ed. 1999;38:2300–2324.Tyler PC, Winchester BG. Iminosugars as Glycosidase Inhibitors. 1999:125–156.Burgess K, Henderson I. Tetrahedron. 1992;48:4045–66.Fellows LE, Kite GC, Nash RJ, Simmonds MSJ, Scofield AM. Rec Adv Phytochem. 1989;23:395–427.
-
-
-
For a review of swainsonine syntheses, see: Nemr AE. Tetrahedron. 2000;56:8579–8629. For more recent syntheses, see: Martin R, Murruzzu C, Pericas MA, Riera A. J Org Chem. 2005;70:2325–2328.Heimgaertner G, Raatz D, Reiser O. Tetrahedron. 2005;61:643–655.Song L, Duesler EN, Mariano PS. J Org Chem. 2004;69:7284–7293.Lindsay KB, Pyne SG. Aus J Chem. 2004;57:669–672.Pearson WH, Ren Y, Powers JD. Heterocycles. 2002;58:421–430.Lindsay KB, Pyne SG. J Org Chem. 2002;67:7774–7780.Buschmann N, Rueckert A, Blechert S. J Org Chem. 2002;67:4325–4329.Zhao H, Hans S, Cheng X, Mootoo DR. J Org Chem. 2001;66:1761–1767.
-
-
- Ali MH, Hough L, Richardson AC. J Chem Soc, Chem Commun. 1984:447–448.
-
- Fleet GWJ, Gough MJ, Smith PW. Tetrahedron Lett. 1984;25:1853–1856.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

