Cortical pain responses in human infants
- PMID: 16597720
- PMCID: PMC6674141
- DOI: 10.1523/JNEUROSCI.0348-06.2006
Cortical pain responses in human infants
Abstract
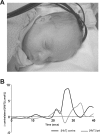
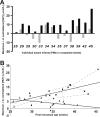
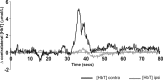
Despite the recent increase in our understanding of the development of pain processing, it is still not known whether premature infants are capable of processing pain at a cortical level. In this study, changes in cerebral oxygenation over the somatosensory cortex were measured in response to noxious stimulation using real-time near-infrared spectroscopy in 18 infants aged between 25 and 45 weeks postmenstrual age. The noxious stimuli were heel lances performed for routine blood sampling; no blood tests were performed solely for the purpose of the study. Noxious stimulation produced a clear cortical response, measured as an increase in total hemoglobin concentration [HbT] in the contralateral somatosensory cortex, from 25 weeks (mean Delta[HbT] = 7.74 micromol/L; SE, 1.10). Cortical responses were significantly greater in awake compared with sleeping infants, with a mean difference of 6.63 micromol/L [95% confidence interval (CI) limits: 2.35, 10.91 micromol/L; mean age, 35.2 weeks]. In awake infants, the response in the contralateral somatosensory cortex increased with age (regression coefficient, 0.698 micromol/L/week; 95% CI limits: 0.132, 1.265 micromol/L/week) and the latency decreased with age (regression coefficient, -0.9861 micromol/L/week; 95% CI limits: -1.5361, -0.4361 micromol/L/week; age range, 25-38 weeks). The response was modality specific because no response was detected after non-noxious stimulation of the heel, even when accompanied by reflex withdrawal of the foot. We conclude that noxious information is transmitted to the preterm infant cortex from 25 weeks, highlighting the potential for both higher-level pain processing and pain-induced plasticity in the human brain from a very early age.
Figures
References
-
- Anand KJ (2000). Pain, plasticity, and premature birth: a prescription for permanent suffering? Nat Med 6:971–973. - PubMed
-
- Andrews K, Fitzgerald M (1994). The cutaneous withdrawal reflex in human neonates: sensitization, receptive fields, and the effects of contralateral stimulation. Pain 56:95–101. - PubMed
-
- Andrews K, Fitzgerald M (1999). Cutaneous flexion reflex in human neonates: a quantitative study of threshold and stimulus-response characteristics after single and repeated stimuli. Dev Med Child Neurol 41:696–703. - PubMed
-
- Andrews KA, Desai D, Dhillon HK, Wilcox DT, Fitzgerald M (2002). Abdominal sensitivity in the first year of life: comparison of infants with and without prenatally diagnosed unilateral hydronephrosis. Pain 100:35–46. - PubMed
-
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005). Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
