Neural variability in premotor cortex provides a signature of motor preparation
- PMID: 16597724
- PMCID: PMC6674116
- DOI: 10.1523/JNEUROSCI.3762-05.2006
Neural variability in premotor cortex provides a signature of motor preparation
Abstract
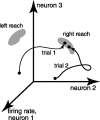
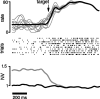
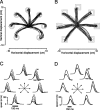
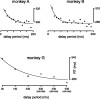
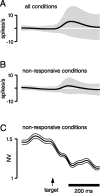
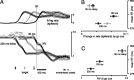
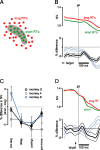
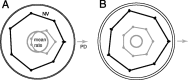
We present experiments and analyses designed to test the idea that firing rates in premotor cortex become optimized during motor preparation, approaching their ideal values over time. We measured the across-trial variability of neural responses in dorsal premotor cortex of three monkeys performing a delayed-reach task. Such variability was initially high, but declined after target onset, and was maintained at a rough plateau during the delay. An additional decline was observed after the go cue. Between target onset and movement onset, variability declined by an average of 34%. This decline in variability was observed even when mean firing rate changed little. We hypothesize that this effect is related to the progress of motor preparation. In this interpretation, firing rates are initially variable across trials but are brought, over time, to their "appropriate" values, becoming consistent in the process. Consistent with this hypothesis, reaction times were longer if the go cue was presented shortly after target onset, when variability was still high, and were shorter if the go cue was presented well after target onset, when variability had fallen to its plateau. A similar effect was observed for the natural variability in reaction time: longer (shorter) reaction times tended to occur on trials in which firing rates were more (less) variable. These results reveal a remarkable degree of temporal structure in the variability of cortical neurons. The relationship with reaction time argues that the changes in variability approximately track the progress of motor preparation.
Figures




References
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A (1996). Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273:1868–1871. - PubMed
-
- Bair W, Koch C (1996). Temporal precision of spike trains in extrastriate cortex of the behaving macaque monkey. Neural Comput 8:1185–1202. - PubMed
-
- Bair W, O'Keefe LP (1998). The influence of fixational eye movements on the response of neurons in area MT of the macaque. Vis Neurosci 15:779–786. - PubMed
-
- Bastian A, Riehle A, Erlhagen W, Schoner G (1998). Prior information preshapes the population representation of movement direction in motor cortex. NeuroReport 9:315–319. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
