The dynamics of spatiotemporal response integration in the somatosensory cortex of the vibrissa system
- PMID: 16597730
- PMCID: PMC6674119
- DOI: 10.1523/JNEUROSCI.4056-05.2006
The dynamics of spatiotemporal response integration in the somatosensory cortex of the vibrissa system
Abstract
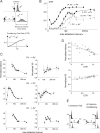
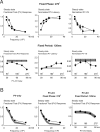
Spatiotemporal response integration across the neural receptive field (RF) is a general feature of sensory coding and has an important role in shaping responses to naturalistic stimuli. In the primary somatosensory cortex of the rat vibrissa pathway, such integration across the vibrissa array strongly shapes the coding of spatiotemporally distributed deflections. Using a spatiotemporal paired-pulse paradigm, this study revealed that fundamentally different types of pairwise interactions have similar qualitative behavior but that the magnitude, latency, and precision of the neural responses depend on the specific RF components being engaged. In all cases, however, increase in the suppression of response magnitude accompanied a lengthening of latency and a decrease in response precision. Furthermore, nonlinear interactions evoked by stimulation of multiple RF subregions strongly influence both response magnitude and timing to more complex sequences. Despite their complexity, such response interactions are highly predictable from elementary pairwise interactions. To understand the functional role of spatiotemporal interactions in coding, we developed a response model that incorporated the experimentally measured modulations in response magnitude, latency, and precision induced by cross-vibrissa interactions. Simulations of a simplified textural discrimination task indicate that spatiotemporal interactions enhance discrimination under certain stimulus time scales. This improvement follows from a nonlinear response property that acts to restore the neural response in the face of suppression. Together, the present findings highlight the role of response integration in shaping single-cell responses and provide predictions about how changes in response parameters influence coding accuracy.
Figures





References
-
- Ahissar E, Sosnik R, Haidarliu S (2000). Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature 406:302–306. - PubMed
-
- Ahissar E, Sosnik R, Bagdasarian K, Haidarliu S (2001). Temporal frequency of whisker movement. II. Laminar organization of cortical representations. J Neurophysiol 86:354–367. - PubMed
-
- Andermann ML, Ritt J, Neimark MA, Moore CI (2004). Neural correlates of vibrissa resonance: band-pass and somatotopic representation of high-frequency stimuli. Neuron 42:451–463. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
