Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival
- PMID: 16597733
- PMCID: PMC6674138
- DOI: 10.1523/JNEUROSCI.5338-05.2006
Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival
Abstract
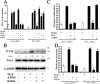
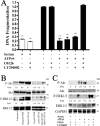
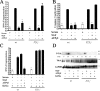
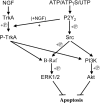
Cell survival is an essential function in the development and maintenance of the nervous system. We demonstrate here a previously unappreciated role for extracellular nucleotide signaling through the P2Y2 receptor in the survival of neurons: PC12 (pheochromocytoma 12) cells and dorsal root ganglion neurons are protected from serum starvation-induced apoptosis by ATP, UTP, and ATPgammaS, an effect mediated via P2Y2 receptors, as demonstrated by small interfering RNA and genetic knock-out models. This protection occurs independently of neurophin signaling but requires Src activation of ERK (extracellular signal-regulated kinase) and Akt. Moreover, ATPgammaS and NGF act synergistically to enhance neuronal survival through enhanced TrkA signaling. The results, which define a novel mechanism for inhibition of apoptosis, implicate parallel, interacting systems--extracellular nucleotides/P2Y2 receptors and neurotrophin/TrkA--to sustain neuronal survival.
Figures


References
-
- Akassoglou K (2005). Nerve growth factor-independent neuronal survival: a role for NO donors. Mol Pharmacol 68:952–955. - PubMed
-
- Bacon KB, Camp RD (1990). Interleukin (IL)-8-induced in vitro human lymphocyte migration is inhibited by cholera and pertussis toxins and inhibitors of protein kinase C. Biochem Biophys Res Commun 169:1099–1104. - PubMed
-
- Berra E, Diaz-Meco MT, Moscat J (1998). The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem 273:10792–10797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
