pK values of the ionizable groups of proteins
- PMID: 16597822
- PMCID: PMC2242523
- DOI: 10.1110/ps.051840806
pK values of the ionizable groups of proteins
Abstract
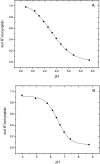
We have used potentiometric titrations to measure the pK values of the ionizable groups of proteins in alanine pentapeptides with appropriately blocked termini. These pentapeptides provide an improved model for the pK values of the ionizable groups in proteins. Our pK values determined in 0.1 M KCl at 25 degrees C are: 3.67+/-0.03 (alpha-carboxyl), 3.67+/-0.04 (Asp), 4.25+/-0.05 (Glu), 6.54+/-0.04 (His), 8.00+/-0.03 (alpha-amino), 8.55+/-0.03 (Cys), 9.84+/-0.11 (Tyr), and 10.40+/-0.08 (Lys). The pK values of some groups differ from the Nozaki and Tanford (N & T) pK values often used in the literature: Asp (3.67 this work vs. 4.0 N & T); His (6.54 this work vs. 6.3 N & T); alpha-amino (8.00 this work vs. 7.5 N & T); Cys (8.55 this work vs. 9.5 N & T); and Tyr (9.84 this work vs. 9.6 N & T). Our pK values will be useful to those who study pK perturbations in folded and unfolded proteins, and to those who use theory to gain a better understanding of the factors that determine the pK values of the ionizable groups of proteins.
Figures
References
-
- Anderson D.E., Becktel W.J., Dahlquist F.W. 1990. pH-induced denaturation of proteins: A single salt bridge contributes 3–5 kcal/mol to the free energy of folding of T4 lysozyme Biochemistry 29 2403–2408. - PubMed
-
- Antosiewicz J., McCammon J.A., Gilson M.K. 1996. The determinants of pKas in proteins Biochemistry 35 7819–7833. - PubMed
-
- Bartlett G.J., Porter C.T., Borkakoti N., Thornton J.M. 2002. Analysis of catalytic residues in enzyme active sites J. Mol. Biol. 324 105–121. - PubMed
-
- Braun-Sand S. and Warshel A. 2005. Electrostatics of proteins: Principles, models, and applications In Protein folding handbook, part I. (eds. Buchner J. and Kiefhaber T.) . . Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim.
-
- Bulaj G., Kortemme T., Goldenberg D.P. 1998. Ionization–reactivity relationships for cysteine thiols in polypeptides Biochemistry 37 8965–8972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources