Affinity enhancement of an in vivo matured therapeutic antibody using structure-based computational design
- PMID: 16597831
- PMCID: PMC2242497
- DOI: 10.1110/ps.052030506
Affinity enhancement of an in vivo matured therapeutic antibody using structure-based computational design
Abstract
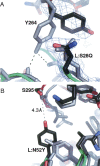
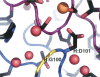
Improving the affinity of a high-affinity protein-protein interaction is a challenging problem that has practical applications in the development of therapeutic biomolecules. We used a combination of structure-based computational methods to optimize the binding affinity of an antibody fragment to the I-domain of the integrin VLA1. Despite the already high affinity of the antibody (Kd approximately 7 nM) and the moderate resolution (2.8 A) of the starting crystal structure, the affinity was increased by an order of magnitude primarily through a decrease in the dissociation rate. We determined the crystal structure of a high-affinity quadruple mutant complex at 2.2 A. The structure shows that the design makes the predicted contacts. Structural evidence and mutagenesis experiments that probe a hydrogen bond network illustrate the importance of satisfying hydrogen bonding requirements while seeking higher-affinity mutations. The large and diverse set of interface mutations allowed refinement of the mutant binding affinity prediction protocol and improvement of the single-mutant success rate. Our results indicate that structure-based computational design can be successfully applied to further improve the binding of high-affinity antibodies.
Figures


Similar articles
-
Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen.J Mol Biol. 1999 Nov 5;293(4):865-81. doi: 10.1006/jmbi.1999.3192. J Mol Biol. 1999. PMID: 10543973
-
IsAb: a computational protocol for antibody design.Brief Bioinform. 2021 Sep 2;22(5):bbab143. doi: 10.1093/bib/bbab143. Brief Bioinform. 2021. PMID: 33876197 Free PMC article.
-
Influence of canonical structure determining residues on antibody affinity and stability.J Struct Biol. 2014 Feb;185(2):223-7. doi: 10.1016/j.jsb.2013.08.009. Epub 2013 Aug 29. J Struct Biol. 2014. PMID: 23994046
-
Computer-Aided Antibody Design: An Overview.Adv Exp Med Biol. 2017;1053:221-243. doi: 10.1007/978-3-319-72077-7_11. Adv Exp Med Biol. 2017. PMID: 29549642 Review.
-
Computational design of antibodies.Curr Opin Struct Biol. 2018 Aug;51:156-162. doi: 10.1016/j.sbi.2018.04.007. Epub 2018 May 20. Curr Opin Struct Biol. 2018. PMID: 29791878 Review.
Cited by
-
Toward real-world automated antibody design with combinatorial Bayesian optimization.Cell Rep Methods. 2023 Jan 3;3(1):100374. doi: 10.1016/j.crmeth.2022.100374. eCollection 2023 Jan 23. Cell Rep Methods. 2023. PMID: 36814835 Free PMC article.
-
Facile Affinity Maturation of Antibody Variable Domains Using Natural Diversity Mutagenesis.Front Immunol. 2017 Sep 4;8:986. doi: 10.3389/fimmu.2017.00986. eCollection 2017. Front Immunol. 2017. PMID: 28928732 Free PMC article.
-
Hallucinating structure-conditioned antibody libraries for target-specific binders.Front Immunol. 2022 Oct 21;13:999034. doi: 10.3389/fimmu.2022.999034. eCollection 2022. Front Immunol. 2022. PMID: 36341416 Free PMC article.
-
Production of Antigen-Binding Fragment against O,O-Diethyl Organophosphorus Pesticides and Molecular Dynamics Simulations of Antibody Recognition.Int J Mol Sci. 2018 May 6;19(5):1381. doi: 10.3390/ijms19051381. Int J Mol Sci. 2018. PMID: 29734787 Free PMC article.
-
OptMAVEn--a new framework for the de novo design of antibody variable region models targeting specific antigen epitopes.PLoS One. 2014 Aug 25;9(8):e105954. doi: 10.1371/journal.pone.0105954. eCollection 2014. PLoS One. 2014. PMID: 25153121 Free PMC article.
References
-
- Batista F.D. and Neuberger M.S. 1998. Affinity dependence of the B cell response to antigen: A threshold, a ceiling, and the importance of off-rate Immunity 8: 751–759. - PubMed
-
- Ben-horin S. and Bank I. 2004. The role of very late antigen-1 in immune-mediated inflammation Clin. Immunol. 113: 119–129. - PubMed
-
- Brekke O.H. and Sandlie I. 2003. Therapeutic antibodies for human diseases at the dawn of the twenty-first century Nat. Rev. Drug Discov. 2: 52–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

