Structure and dynamics of the epidermal growth factor receptor C-terminal phosphorylation domain
- PMID: 16597832
- PMCID: PMC2242510
- DOI: 10.1110/ps.052045306
Structure and dynamics of the epidermal growth factor receptor C-terminal phosphorylation domain
Abstract
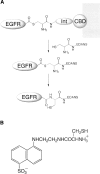
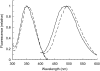
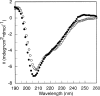
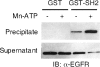
The C-terminal phosphorylation domain of the epidermal growth factor receptor is believed to regulate protein kinase activity as well as mediate the assembly of signal transduction complexes. The structure and dynamics of this proposed autoregulatory domain were examined by labeling the extreme C terminus of the EGFR intracellular domain (ICD) with an extrinsic fluorophore. Fluorescence anisotropy decay analysis of the nonphosphorylated EGFR-ICD yielded two rotational correlation times: a longer time, consistent with the global rotational motion of a 60- to 70-kDa protein with an elongated globular conformation, and a shorter time, presumably contributed by segmental motion near the fluorophore. A C-terminally truncated form of EGFR-ICD yielded a slow component consistent with the rotational motion of the 38-kDa kinase core. These findings suggested a structural arrangement of the EGFR-ICD in which the C-terminal phosphorylation domain interacts with the kinase core to move as an extended structure. A marked reduction in the larger correlation time of EGFR-ICD was observed upon its autophosphorylation. This dynamic component was faster than predicted for the globular motion of the 62-kDa EGFR-ICD, suggesting an increase in the mobility of the C-terminal domain and a likely displacement of this domain from the kinase core. The interaction between the SH2 domain of c-Src and the phosphorylated EGFR C-terminal domain was shown to impede its mobility. Circular dichroism spectroscopy indicated that the EGFR C-terminal domain possessed a significant level of secondary structure in the form of alpha-helices and beta-sheets, with a marginal change in beta-sheet content occurring upon phosphorylation.
Figures




References
-
- Bertics P.J. and Gill G.N. 1985. Self-phosphorylation enhances the protein-tyrosine kinase activity of the epidermal growth factor receptor J. Biol. Chem. 260: 14642–14647. - PubMed
-
- Bertics P.J., Chen W.S., Hubler L., Lazar C.S., Rosenfeld M.G., Gill G.N. 1988. Alteration of epidermal growth factor receptor activity by mutation of its primary carboxyl-terminal site of tyrosine self-phosphorylation J. Biol. Chem. 263: 3610–3617. - PubMed
-
- Burgess A.W., Cho H.S., Eigenbrot C., Ferguson K.M., Garrett T.P., Leahy D.J., Lemmon M.A., Sliwkowski M.X., Ward C.W., Yokoyama S. 2003. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors Mol. Cell 12: 541–552. - PubMed
-
- Cadena D.L., Chan C.L., Gill G.N. 1994. The intracellular tyrosine kinase domain of the epidermal growth factor receptor undergoes a conformational change upon autophosphorylation J. Biol. Chem. 269: 260–265. - PubMed
-
- Cheng K. and Koland J.G. 1996. Nucleotide binding by the epidermal growth factor receptor protein-tyrosine kinase. Trinitrophenyl-ATP as a spectroscopic probe J. Biol. Chem. 271: 311–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

