Rapid detection of norovirus from fecal specimens by real-time reverse transcription-loop-mediated isothermal amplification assay
- PMID: 16597865
- PMCID: PMC1448634
- DOI: 10.1128/JCM.44.4.1376-1381.2006
Rapid detection of norovirus from fecal specimens by real-time reverse transcription-loop-mediated isothermal amplification assay
Abstract
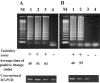
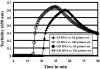
In this study, we developed a one-step, single-tube genogroup-specific reverse transcription-loop-mediated isothermal amplification (RT-LAMP) assay for the detection of norovirus (NoV) genomes targeting from the C terminus of the RNA-dependent RNA polymerase gene to the capsid N-terminal/shell domain region. This is the first report on the development of an RT-LAMP assay for the detection of NoV genomes. Because of the diversity of NoV genotypes, we used 9 and 13 specially designed primers containing mixed bases for genogroup I (GI) and II (GII), respectively. The RT-LAMP assay had the advantages of rapidity, simplicity, specificity, and selectively and could obtain results within 90 min, generally even within 60 min, under isothermal conditions at 62 degrees C. The detection limits for NoV genomes were between 10(2) and 10(3) copies/tube for GI and GII with differentiation by genotype, and no cross-reactions among NoV GI and GII and other gastroenteritis viruses, such as sapovirus, human astrovirus, adenovirus type 40 and 41, and group A and C rotavirus, were found. In the evaluation tests with fecal specimens obtained from gastroenteritis outbreaks, the sensitivity and specificity of the RT-LAMP assay with regard to RT-PCR were 100 and 94% for GI and 100 and 100% for GII, respectively. These findings establish that the RT-LAMP assay is potentially useful for the rapid detection of NoV genomes from fecal specimens in outbreaks of food-borne and person-to-person-transmitted gastroenteritis.
Figures

References
-
- Bon, F., K. Ambert-Balay, H. Giraudon, J. Kaplon, S. Le Guyader, M. Pommepuy, A. Gallay, V. Vaillant, H. de Valk, R. Chikhi-Brachet, A. Flahaut, P. Pothier, and E. Kohli. 2005. Molecular epidemiology of caliciviruses detected in sporadic and outbreak cases of gastroenteritis in France from December 1998 to February 2004. J. Clin. Microbiol. 43:4659-4664. - PMC - PubMed
-
- Cheng, P. K. C., D. K. K. Wong, T. W. H. Chung, and W. W. L. Lim. 2005. Norovirus contamination found in oysters worldwide. J. Med. Virol. 76:593-597. - PubMed
-
- Fankhauser, R. L., S. S. Monroe, J. S. Noel, C. D. Humphrey, J. S. Bresee, U. D. Parashar, T. Ando, and R. I. Glass. 2002. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 186:1-7. - PubMed
-
- Green, K. Y., R. M. Chanock, and A. Z. Kapikian. 2001. Human caliciviruses, p. 841-874. In D. M. Knipe, P. M. Howley, D. E. Griffin, et al. (ed.), Fields virology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

