The phosphopantetheinyl transferase superfamily: phylogenetic analysis and functional implications in cyanobacteria
- PMID: 16597923
- PMCID: PMC1449050
- DOI: 10.1128/AEM.72.4.2298-2305.2006
The phosphopantetheinyl transferase superfamily: phylogenetic analysis and functional implications in cyanobacteria
Abstract
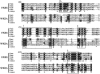
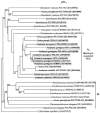
Phosphopantetheinyl transferases (PPTs) are a superfamily of essential enzymes required for the synthesis of a wide range of compounds including fatty acid, polyketide, and nonribosomal peptide metabolites. These enzymes activate carrier proteins in specific biosynthetic pathways by the transfer of a phosphopantetheinyl moiety to an invariant serine residue. PPTs display low levels of sequence similarity but can be classified into two major families based on several short motifs. The prototype of the first family is the broad-substrate-range PPT Sfp, which is required for biosynthesis of surfactin in Bacillus subtilis. The second family is typified by the Escherichia coli acyl carrier protein synthase (AcpS). Facilitated by the growing number of genome sequences available for analyses, large-scale phylogenetic studies were utilized in this research to reveal novel subfamily groupings, including two subfamilies within the Sfp-like family. In the present study degenerate oligonucleotide primers were designed for amplification of cyanobacterial PPT gene fragments. Subsequent phylogenetic analyses suggested a unique, function-based PPT type, defined by the PPTs involved in heterocyst differentiation. Evidence supporting this hypothesis was obtained by sequencing the region surrounding the partial Nodularia spumigena PPT gene. The ability to genetically classify PPT function is critical for the engineering of novel compounds utilizing combinatorial biosynthesis techniques. Information regarding cyanobacterial PPTs has important ramifications for the ex situ production of cyanobacterial natural products.
Figures
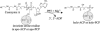

Similar articles
-
Characterization of PPTNs, a cyanobacterial phosphopantetheinyl transferase from Nodularia spumigena NSOR10.J Bacteriol. 2007 Apr;189(8):3133-9. doi: 10.1128/JB.01850-06. Epub 2007 Feb 16. J Bacteriol. 2007. PMID: 17307858 Free PMC article.
-
Distribution and functional analysis of the phosphopantetheinyl transferase superfamily in Actinomycetales microorganisms.Proc Natl Acad Sci U S A. 2018 Jun 26;115(26):6828-6833. doi: 10.1073/pnas.1800715115. Epub 2018 Jun 14. Proc Natl Acad Sci U S A. 2018. PMID: 29903901 Free PMC article.
-
An In Vitro and In Vivo Study of Broad-Range Phosphopantetheinyl Transferases for Heterologous Expression of Cyanobacterial Natural Products.ACS Synth Biol. 2018 Apr 20;7(4):1143-1151. doi: 10.1021/acssynbio.8b00091. Epub 2018 Mar 27. ACS Synth Biol. 2018. PMID: 29562128
-
Fluorescent site-specific labeling of Escherichia coli expressed proteins with Sfp phosphopantetheinyl transferase.Methods Mol Biol. 2011;705:295-307. doi: 10.1007/978-1-61737-967-3_18. Methods Mol Biol. 2011. PMID: 21125394 Review.
-
Post-translational modification of polyketide and nonribosomal peptide synthases.Curr Opin Chem Biol. 1997 Oct;1(3):309-15. doi: 10.1016/s1367-5931(97)80067-1. Curr Opin Chem Biol. 1997. PMID: 9667867 Review.
Cited by
-
Cyanobacterial Sfp-type phosphopantetheinyl transferases functionalize carrier proteins of diverse biosynthetic pathways.Sci Rep. 2017 Sep 19;7(1):11888. doi: 10.1038/s41598-017-12244-3. Sci Rep. 2017. PMID: 28928426 Free PMC article.
-
Trujillonella humicola sp. nov., a siderophore-synthesizing bacterium isolated from black soil in Northeast China.Antonie Van Leeuwenhoek. 2025 Apr 22;118(5):73. doi: 10.1007/s10482-025-02081-0. Antonie Van Leeuwenhoek. 2025. PMID: 40261431
-
The phosphopantetheinyl transferases: catalysis of a post-translational modification crucial for life.Nat Prod Rep. 2014 Jan;31(1):61-108. doi: 10.1039/c3np70054b. Nat Prod Rep. 2014. PMID: 24292120 Free PMC article. Review.
-
Mining of Cyanobacterial Genomes Indicates Natural Product Biosynthetic Gene Clusters Located in Conjugative Plasmids.Front Microbiol. 2021 Nov 4;12:684565. doi: 10.3389/fmicb.2021.684565. eCollection 2021. Front Microbiol. 2021. PMID: 34803938 Free PMC article.
-
Ces locus embedded proteins control the non-ribosomal synthesis of the cereulide toxin in emetic Bacillus cereus on multiple levels.Front Microbiol. 2015 Oct 13;6:1101. doi: 10.3389/fmicb.2015.01101. eCollection 2015. Front Microbiol. 2015. PMID: 26528255 Free PMC article.
References
-
- Burja, A. M., B. Banaigs, E. Abou-Mansour, J. G. Burgess, and P. C. Wright. 2001. Marine cyanobacteria: a prolific source of natural products. Tetrahedron 57:9347-9377.
-
- Caffrey, P., B. Green, L. C. Packman, B. J. Rawlings, J. Staunton, and P. F. Leadlay. 1991. An acyl-carrier-protein-thioesterase domain from the 6-deoxyerythronolide B synthase of Saccharopolyspora erythraea: high-level production, purification and characterisation in Escherichia coli. Eur. J. Biochem. 195:823-830. - PubMed
-
- Campbell, E. L., M. F. Cohen, and J. C. Meeks. 1997. A polyketide-synthase-like gene is involved in the synthesis of heterocyst glycolipids in Nostoc punctiforme strain ATCC 29133. Arch. Microbiol. 167:251-258. - PubMed
-
- Cane, D. E., C. T. Walsh, and C. Khosla. 1998. Harnessing the biosynthetic code: combinations, permutations, and mutations. Science 282:63-68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

