Bioenergetic mechanism for nisin resistance, induced by the acid tolerance response of Listeria monocytogenes
- PMID: 16597957
- PMCID: PMC1449014
- DOI: 10.1128/AEM.72.4.2556-2563.2006
Bioenergetic mechanism for nisin resistance, induced by the acid tolerance response of Listeria monocytogenes
Abstract
This study examined the bioenergetics of Listeria monocytogenes, induced to an acid tolerance response (ATR). Changes in bioenergetic parameters were consistent with the increased resistance of ATR-induced (ATR(+)) cells to the antimicrobial peptide nisin. These changes may also explain the increased resistance of L. monocytogenes to other lethal factors. ATR(+) cells had lower transmembrane pH (DeltapH) and electric potential (Deltapsi) than the control (ATR(-)) cells. The decreased proton motive force (PMF) of ATR(+) cells increased their resistance to nisin, the action of which is enhanced by energized membranes. Paradoxically, the intracellular ATP levels of the PMF-depleted ATR(+) cells were approximately 7-fold higher than those in ATR(-) cells. This suggested a role for the F(o)F(1) ATPase enzyme complex, which converts the energy of ATP hydrolysis to PMF. Inhibition of the F(o)F(1) ATPase enzyme complex by N'-N'-1,3-dicyclohexylcarbodiimide increased ATP levels in ATR(-) but not in ATR(+) cells, where ATPase activity was already low. Spectrometric analyses (surface-enhanced laser desorption ionization-time of flight mass spectrometry) suggested that in ATR(+) listeriae, the downregulation of the proton-translocating c subunit of the F(o)F(1) ATPase was responsible for the decreased ATPase activity, thereby sparing vital ATP. These data suggest that regulation of F(o)F(1) ATPase plays an important role in the acid tolerance response of L. monocytogenes and in its induced resistance to nisin.
Figures

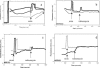
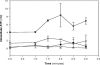


References
-
- Bonnet, M., and T. J. Montville. 2005. Acid-tolerant Listeria monocytogenes persist in a model food system fermented with nisin-producing bacteria. Lett. Appl. Microbiol. 40:237-242. - PubMed
-
- Boyer, P. D. 1997. The ATP synthase—a splendid molecular machine. Annu. Rev. Biochem. 66:717-749. - PubMed
-
- Breukink, E., and B. de Kruijff. 1999. The lantibiotic nisin, a special case or not? Biochim. Biophys. Acta 1462:223-234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

