Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA, and solar disinfection
- PMID: 16597961
- PMCID: PMC1449012
- DOI: 10.1128/AEM.72.4.2586-2593.2006
Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA, and solar disinfection
Abstract
Knowledge about the sensitivity of the test organism is essential for the evaluation of any disinfection method. In this work we show that sensitivity of Escherichia coli MG1655 to three physical stresses (mild heat, UVA light, and sunlight) that are relevant in the disinfection of drinking water with solar radiation is determined by the specific growth rate of the culture. Batch- and chemostat-cultivated cells from cultures with similar specific growth rates showed similar stress sensitivities. Generally, fast-growing cells were more sensitive to the stresses than slow-growing cells. For example, slow-growing chemostat-cultivated cells (D = 0.08 h(-1)) and stationary-phase bacteria from batch culture that were exposed to mild heat had very similar T(90) (time until 90% of the population is inactivated) values (T(90, chemostat) = 2.66 h; T(90, batch) = 2.62 h), whereas T(90) for cells growing at a mu of 0.9 h(-1) was 0.2 h. We present evidence that the stress sensitivity of E. coli is correlated with the intracellular level of the alternative sigma factor RpoS. This is also supported by the fact that E. coli rpoS mutant cells were more stress sensitive than the parent strain by factors of 4.9 (mild heat), 5.3 (UVA light), and 4.1 (sunlight). Furthermore, modeling of inactivation curves with GInaFiT revealed that the shape of inactivation curves changed depending on the specific growth rate. Inactivation curves of cells from fast-growing cultures (mu = 1.0 h(-1)) that were irradiated with UVA light showed a tailing effect, while for slow-growing cultures (mu = 0.3 h(-1)), inactivation curves with shoulders were obtained. Our findings emphasize the need for accurate reporting of specific growth rates and detailed culture conditions in disinfection studies to allow comparison of data from different studies and laboratories and sound interpretation of the data obtained.
Figures
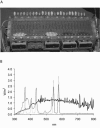
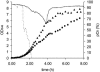


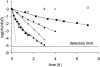
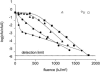
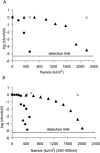
 , 0.6 h−1[▴], and 0.9 h−1[•]). Curves were fitted with GInaFiT (14). The best fit is displayed for each curve. Inactivation curves from Fig. 4 are displayed as dashed lines for comparison. The values for unstressed control samples are displayed by open symbols. Sensitivity was determined as CFU/CFU at time zero.
, 0.6 h−1[▴], and 0.9 h−1[•]). Curves were fitted with GInaFiT (14). The best fit is displayed for each curve. Inactivation curves from Fig. 4 are displayed as dashed lines for comparison. The values for unstressed control samples are displayed by open symbols. Sensitivity was determined as CFU/CFU at time zero.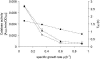


References
-
- Acra, A., Z. Raffoul, and Y. Karahagopian. 1984. Solar disinfection of drinking water and oral rehydration solutions. United Nations Children's Fund, New York, N.Y.
-
- Allwood, M. C., and A. D. Russell. 1970. Mechanisms of thermal injury in nonsporulating bacteria. Adv. Appl. Microbiol. 12:89-119. - PubMed
-
- Barry, V. C., M. L. Conalty, J. M. Denneny, and F. Winder. 1956. Peroxide formation in bacteriological media. Nature 178:596-597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

