Toxicity and mode of action of Bacillus thuringiensis Cry proteins in the Mediterranean corn borer, Sesamia nonagrioides (Lefebvre)
- PMID: 16597962
- PMCID: PMC1449080
- DOI: 10.1128/AEM.72.4.2594-2600.2006
Toxicity and mode of action of Bacillus thuringiensis Cry proteins in the Mediterranean corn borer, Sesamia nonagrioides (Lefebvre)
Abstract
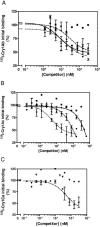
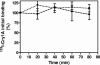
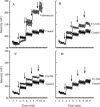
Sesamia nonagrioides is one of the most damaging pests of corn in Spain and other Mediterranean countries. Bt corn expressing the Bacillus thuringiensis Cry1Ab toxin is being grown on about 58,000 ha in Spain. Here we studied the mode of action of this Cry protein on S. nonagrioides (binding to specific receptors, stability of binding, and pore formation) and the modes of action of other Cry proteins that were found to be active in this work (Cry1Ac, Cry1Ca, and Cry1Fa). Binding assays were performed with (125)I- or biotin-labeled toxins and larval brush border membrane vesicles (BBMV). Competition experiments indicated that these toxins bind specifically and that Cry1Aa, Cry1Ab, and Cry1Ac share a binding site. Cry1Ca and Cry1Fa bind to different sites. In addition, Cry1Fa binds to Cry1A's binding site with very low affinity and vice versa. Binding of Cry1Ab and Cry1Ac was found to be stable over time, which indicates that the observed binding is irreversible. The pore-forming activity of Cry proteins on BBMV was determined using the voltage-sensitive fluorescent dye DiSC(3)(5). Membrane permeability increased in the presence of the active toxins Cry1Ab and Cry1Fa but not in the presence of the nonactive toxin Cry1Da. In terms of resistance management, based on our results and the fact that Cry1Ca is not toxic to Ostrinia nubilalis, we recommend pyramiding of Cry1Ab with Cry1Fa in the same Bt corn plant for better long-term control of corn borers.
Figures

Similar articles
-
Leucine transport is affected by Bacillus thuringiensis Cry1 toxins in brush border membrane vesicles from Ostrinia nubilalis Hb (Lepidoptera: Pyralidae) and Sesamia nonagrioides Lefebvre (Lepidoptera: Noctuidae) midgut.J Membr Biol. 2006;214(3):157-64. doi: 10.1007/s00232-006-0042-1. Epub 2007 Jun 8. J Membr Biol. 2006. PMID: 17558532
-
Use of Bacillus thuringiensis toxins for control of the cotton pest Earias insulana (Boisd.) (Lepidoptera: Noctuidae).Appl Environ Microbiol. 2006 Jan;72(1):437-42. doi: 10.1128/AEM.72.1.437-442.2006. Appl Environ Microbiol. 2006. PMID: 16391075 Free PMC article.
-
Toxicity and Binding Studies of Bacillus thuringiensis Cry1Ac, Cry1F, Cry1C, and Cry2A Proteins in the Soybean Pests Anticarsia gemmatalis and Chrysodeixis (Pseudoplusia) includens.Appl Environ Microbiol. 2017 May 17;83(11):e00326-17. doi: 10.1128/AEM.00326-17. Print 2017 Jun 1. Appl Environ Microbiol. 2017. PMID: 28363958 Free PMC article.
-
Bt maize and integrated pest management--a European perspective.Pest Manag Sci. 2011 Sep;67(9):1049-58. doi: 10.1002/ps.2221. Epub 2011 Jun 27. Pest Manag Sci. 2011. PMID: 21710684 Review.
-
Effects and mechanisms of Bacillus thuringiensis crystal toxins for mosquito larvae.Insect Sci. 2017 Oct;24(5):714-729. doi: 10.1111/1744-7917.12401. Epub 2016 Nov 24. Insect Sci. 2017. PMID: 27628909 Review.
Cited by
-
Bacillus thuringiensis Cry1Ac toxin-binding and pore-forming activity in brush border membrane vesicles prepared from anterior and posterior midgut regions of lepidopteran larvae.Appl Environ Microbiol. 2008 Mar;74(6):1710-6. doi: 10.1128/AEM.02827-07. Epub 2008 Jan 25. Appl Environ Microbiol. 2008. PMID: 18223107 Free PMC article.
-
Bitrophic and tritrophic effects of Bt Cry3A transgenic potato on beneficial, non-target, beetles.Transgenic Res. 2007 Dec;16(6):795-812. doi: 10.1007/s11248-007-9088-9. Epub 2007 Apr 6. Transgenic Res. 2007. PMID: 17415673
-
Insect Resistance to Bacillus thuringiensis Toxin Cry2Ab Is Conferred by Mutations in an ABC Transporter Subfamily A Protein.PLoS Genet. 2015 Nov 19;11(11):e1005534. doi: 10.1371/journal.pgen.1005534. eCollection 2015 Nov. PLoS Genet. 2015. PMID: 26583651 Free PMC article.
-
Leucine transport is affected by Bacillus thuringiensis Cry1 toxins in brush border membrane vesicles from Ostrinia nubilalis Hb (Lepidoptera: Pyralidae) and Sesamia nonagrioides Lefebvre (Lepidoptera: Noctuidae) midgut.J Membr Biol. 2006;214(3):157-64. doi: 10.1007/s00232-006-0042-1. Epub 2007 Jun 8. J Membr Biol. 2006. PMID: 17558532
-
Monitoring Insect Resistance to Bt Maize in the European Union: Update, Challenges, and Future Prospects.J Econ Entomol. 2023 Apr 24;116(2):275-288. doi: 10.1093/jee/toac154. J Econ Entomol. 2023. PMID: 36610405 Free PMC article.
References
-
- Azuma, M., and O. Yamashita. 1985. Cellular localization and proposed function of midgut trehalase in the silkworm larva, Bombyx mori. Tissue Cell 17:539-551. - PubMed
-
- Bates, S. L., J. Z. Zhao, R. T. Roush, and A. M. Shelton. 2005. Insect resistance management in GM crops: past, present and future. Nat. Biotechnol. 23:57-62. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Bravo, A., R. Miranda, I. Gómez, and M. Soberón. 2002. Pore formation activity of Cry1Ab toxin from Bacillus thuringiensis in an improved membrane vesicle preparation from Manduca sexta midgut cell microvilli. Biochim. Biophys. Acta 1562:63-69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

