Both msa genes in Renibacterium salmoninarum are needed for full virulence in bacterial kidney disease
- PMID: 16597972
- PMCID: PMC1449061
- DOI: 10.1128/AEM.72.4.2672-2678.2006
Both msa genes in Renibacterium salmoninarum are needed for full virulence in bacterial kidney disease
Abstract
Renibacterium salmoninarum, a gram-positive diplococcobacillus that causes bacterial kidney disease among salmon and trout, has two chromosomal loci encoding the major soluble antigen (msa) gene. Because the MSA protein is widely suspected to be an important virulence factor, we used insertion-duplication mutagenesis to generate disruptions of either the msa1 or msa2 gene. Surprisingly, expression of MSA protein in broth cultures appeared unaffected. However, the virulence of either mutant in juvenile chinook salmon (Oncorhynchus tshawytscha) by intraperitoneal challenge was severely attenuated, suggesting that disruption of the msa1 or msa2 gene affected in vivo expression.
Figures



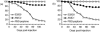
References
-
- Alcorn, S., A. L. Murray, R. J. Pascho, and J. Varney. 2005. A cohabitation challenge to compare the efficacies of vaccines for bacterial kidney disease (BKD) in chinook salmon Oncorhynchus tshawytscha. Dis. Aquat. Organ. 63:151-160. - PubMed
-
- Balfry, S. K., L. J. Albright, and T. P. T. Evelyn. 1996. Horizontal transfer of Renibacterium salmoninarum among farmed salmonids via the fecal-oral route. Dis. Aquat. Organ. 25:1-2.
-
- Brown, L. L., G. K. Iwama, and T. P. T. Evelyn. 1996. The effect of early exposure of coho salmon (Oncorhynchus kisutch) eggs to the p57 protein of Renibacterium salmoninarum on the development of immunity to the pathogen. Fish Shellfish Immunol. 6:149-165.
-
- Bruno, D. W. 2004. Prevalence and diagnosis of bacterial kidney disease (BKD) in Scotland between 1990 and 2002. Dis. Aquat. Organ. 59:125-130. - PubMed
-
- Bruno, D. W. 1988. The relationship between auto-agglutination, cell surface hydrophobicity and virulence of the fish pathogen Renibacterium salmoninarum. FEMS Microbiol. Lett. 51:135-140.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

