Lipid II-based antimicrobial activity of the lantibiotic plantaricin C
- PMID: 16597986
- PMCID: PMC1449081
- DOI: 10.1128/AEM.72.4.2809-2814.2006
Lipid II-based antimicrobial activity of the lantibiotic plantaricin C
Abstract
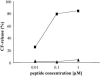
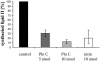
We analyzed the mode of action of the lantibiotic plantaricin C (PlnC), produced by Lactobacillus plantarum LL441. Compared to the well-characterized type A lantibiotic nisin and type B lantibiotic mersacidin, which are both able to interact with the cell wall precursor lipid II, PlnC displays structural features of both prototypes. In this regard, we found that lipid II plays a key role in the antimicrobial activity of PlnC besides that of pore formation. The pore forming activity of PlnC in whole cells was prevented by shielding lipid II on the cell surface. However, in contrast to nisin, PlnC was not able to permeabilize Lactococcus lactis cells or to form pores in 1,2-dioleoyl-sn-glycero-3-phosphocholine liposomes supplemented with 0.1 mol% purified lipid II. This emphasized the different requirements of these lantibiotics for pore formation. Using cell wall synthesis assays, we identified PlnC as a potent inhibitor of (i) lipid II synthesis and (ii) the FemX reaction, i.e., the addition of the first Gly to the pentapeptide side chain of lipid II. As revealed by thin-layer chromatography, both reactions were clearly blocked by the formation of a PlnC-lipid I and/or PlnC-lipid II complex. On the basis of the in vivo and in vitro activities of PlnC shown in this study and the structural lipid II binding motifs described for other lantibiotics, the specific interaction of PlnC with lipid II is discussed.
Figures
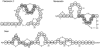


Similar articles
-
Interaction of type A lantibiotics with undecaprenol-bound cell envelope precursors.Microb Drug Resist. 2012 Jun;18(3):261-70. doi: 10.1089/mdr.2011.0242. Epub 2012 Mar 20. Microb Drug Resist. 2012. PMID: 22432708
-
The mode of action of the lantibiotic lacticin 3147--a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II.Mol Microbiol. 2006 Jul;61(2):285-96. doi: 10.1111/j.1365-2958.2006.05223.x. Epub 2006 Jun 12. Mol Microbiol. 2006. PMID: 16771847
-
An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II.Science. 2006 Sep 15;313(5793):1636-7. doi: 10.1126/science.1129818. Science. 2006. PMID: 16973881
-
Antimicrobial mechanism of lantibiotics.Biochem Soc Trans. 2012 Dec 1;40(6):1528-33. doi: 10.1042/BST20120190. Biochem Soc Trans. 2012. PMID: 23176511 Review.
-
Mode of action of lipid II-targeting lantibiotics.Int J Food Microbiol. 2005 May 25;101(2):201-16. doi: 10.1016/j.ijfoodmicro.2004.11.007. Int J Food Microbiol. 2005. PMID: 15862882 Review.
Cited by
-
Cell Wall-active Bacteriocins and Their Applications Beyond Antibiotic Activity.Probiotics Antimicrob Proteins. 2012 Dec;4(4):259-72. doi: 10.1007/s12602-012-9116-9. Probiotics Antimicrob Proteins. 2012. PMID: 26782186
-
Non-proteinogenic amino acids in lacticin 481 analogues result in more potent inhibition of peptidoglycan transglycosylation.ACS Chem Biol. 2012 Nov 16;7(11):1791-5. doi: 10.1021/cb300372b. Epub 2012 Sep 4. ACS Chem Biol. 2012. PMID: 22920239 Free PMC article.
-
In Vitro Antimicrobial Activity and Probiotic Potential of Bifidobacterium and Lactobacillus against Species of Clostridium.Nutrients. 2019 Feb 21;11(2):448. doi: 10.3390/nu11020448. Nutrients. 2019. PMID: 30795551 Free PMC article.
-
Cell wall homeostasis in lactic acid bacteria: threats and defences.FEMS Microbiol Rev. 2020 Sep 1;44(5):538-564. doi: 10.1093/femsre/fuaa021. FEMS Microbiol Rev. 2020. PMID: 32495833 Free PMC article. Review.
-
Mixed Culture Cultivation in Microbial Bioprocesses.Adv Biochem Eng Biotechnol. 2025;189:9-69. doi: 10.1007/10_2023_248. Adv Biochem Eng Biotechnol. 2025. PMID: 38418582 Review.
References
-
- Bierbaum, G., and H. G. Sahl. 1985. Induction of autolysis of staphylococci by the basic peptide antibiotic Pep5 and nisin and their influence on the activity of autolytic enzymes. Arch. Microbiol. 141:249-254. - PubMed
-
- Bonelli, R. R., I. Wiedemann, and H. G. Sahl. Lantibiotics, including nisin. In A. J. Kastin (ed.), Handbook of biologically active peptides, in press. Elsevier Inc., San Diego, Calif.
-
- Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H. G. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. - PubMed
-
- Brötz, H., G. Bierbaum, P. E. Reynolds, and H. G. Sahl. 1997. The lantibiotic mersacidin inhibits peptidoglycan biosynthesis at the level of transglycosylation. Eur. J. Biochem. 246:193-199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

