Peptidoglycan hydrolase fusions maintain their parental specificities
- PMID: 16598006
- PMCID: PMC1448998
- DOI: 10.1128/AEM.72.4.2988-2996.2006
Peptidoglycan hydrolase fusions maintain their parental specificities
Abstract
The increased incidence of bacterial antibiotic resistance has led to a renewed search for novel antimicrobials. Avoiding the use of broad-range antimicrobials through the use of specific peptidoglycan hydrolases (endolysins) might reduce the incidence of antibiotic-resistant pathogens worldwide. Staphylococcus aureus and Streptococcus agalactiae are human pathogens and also cause mastitis in dairy cattle. The ultimate goal of this work is to create transgenic cattle that are resistant to mastitis through the expression of an antimicrobial protein(s) in their milk. Toward this end, two novel antimicrobials were produced. The (i) full-length and (ii) 182-amino-acid, C-terminally truncated S. agalactiae bacteriophage B30 endolysins were fused to the mature lysostaphin protein of Staphylococcus simulans. Both fusions display lytic specificity for streptococcal pathogens and S. aureus. The full lytic ability of the truncated B30 protein also suggests that the SH3b domain at the C terminus is dispensable. The fusions are active in a milk-like environment. They are also active against some lactic acid bacteria used to make cheese and yogurt, but their lytic activity is destroyed by pasteurization (63 degrees C for 30 min). Immunohistochemical studies indicated that the fusion proteins can be expressed in cultured mammalian cells with no obvious deleterious effects on the cells, making it a strong candidate for use in future transgenic mice and cattle. Since the fusion peptidoglycan hydrolase also kills multiple human pathogens, it also may prove useful as a highly selective, multipathogen-targeting antimicrobial agent that could potentially reduce the use of broad-range antibiotics in fighting clinical infections.
Figures

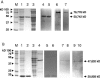
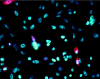

References
-
- Bateman, A., and N. D. Rawlings. 2003. The CHAP domain: a large family of amidases including GSP amidase and peptidoglycan hydrolases. Trends Biochem. Sci. 28:234-237. - PubMed
-
- Bernhardt, T. G., I. N. Wang, D. K. Struck, and R. Young. 2002. Breaking free: “protein antibiotics” and phage lysis. Res. Microbiol. 153:493-501. - PubMed
-
- Bhakta, M., S. Arora, and M. Bal. 2003. Intraspecies transfer of a chloramphenicol-resistance plasmid of staphylococcal origin. Indian J. Med. Res. 117:146-151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

