Mitochondrial glutathione transport: physiological, pathological and toxicological implications
- PMID: 16600197
- PMCID: PMC1621086
- DOI: 10.1016/j.cbi.2006.03.001
Mitochondrial glutathione transport: physiological, pathological and toxicological implications
Abstract
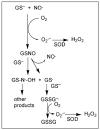
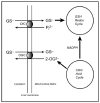
Although most cellular glutathione (GSH) is in the cytoplasm, a distinctly regulated pool is present in mitochondria. Inasmuch as GSH synthesis is primarily restricted to the cytoplasm, the mitochondrial pool must derive from transport of cytoplasmic GSH across the mitochondrial inner membrane. Early studies in liver mitochondria primarily focused on the relationship between GSH status and membrane permeability and energetics. Because GSH is an anion at physiological pH, this suggested that some of the organic anion carriers present in the inner membrane could function in GSH transport. Indeed, studies by Lash and colleagues in isolated mitochondria from rat kidney showed that most of the transport (>80%) in that tissue could be accounted for by function of the dicarboxylate carrier (DIC, Slc25a10) and the oxoglutarate carrier (OGC, Slc25a11), which mediate electroneutral exchange of dicarboxylates for inorganic phosphate and 2-oxoglutarate for other dicarboxylates, respectively. The identity and function of specific carrier proteins in other tissues is less certain, although the OGC is expressed in heart, liver, and brain and the DIC is expressed in liver and kidney. An additional carrier that transports 2-oxoglutarate, the oxodicarboxylate or oxoadipate carrier (ODC; Slc25a21), has been described in rat and human liver and its expression has a wide tissue distribution, although its potential function in GSH transport has not been investigated. Overexpression of the cDNA for the DIC and OGC in a renal proximal tubule-derived cell line, NRK-52E cells, showed that enhanced carrier expression and activity protects against oxidative stress and chemically induced apoptosis. This has implications for development of novel therapeutic approaches for treatment of human diseases and pathological states. Several conditions, such as alcoholic liver disease, cirrhosis or other chronic biliary obstructive diseases, and diabetic nephropathy, are associated with depletion or oxidation of the mitochondrial GSH pool in liver or kidney.
Figures


References
-
- McKernan TM, Woods EB, Lash LH. Uptake of glutathione by renal cortical mitochondria. Arch Biochem Biophys. 1991;288:653–663. - PubMed
-
- Hinchman CA, Ballatori N. Glutathione-degrading capacities of liver and kidney in different species. Biochem Pharmacol. 1990;40:1131–1135. - PubMed
-
- D. P. Jones, L. H. Lash. Introduction: Criteria for assessing normal and abnormal mitochondrial function, in: L.H. Lash, D.P. Jones (Eds.), Mitochondrial Dysfunction, Methods in Toxicology, vol. 2, Academic Press, San Diego, 1993, pp. 1–7.
-
- Hunter FE, Jr, Scott A, Hoffsten PE, Gebicki JM, Weinstein J, Schneider A. Studies on the mechanism of swelling, lysis, and disintegration of isolated liver mitochondria exposed to mixtures of oxidized and reduced glutathione. J Biol Chem. 1964;239:614–621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

