Thyroid-specific enhancer-binding protein/NKX2.1 is required for the maintenance of ordered architecture and function of the differentiated thyroid
- PMID: 16601074
- PMCID: PMC2588428
- DOI: 10.1210/me.2005-0327
Thyroid-specific enhancer-binding protein/NKX2.1 is required for the maintenance of ordered architecture and function of the differentiated thyroid
Abstract
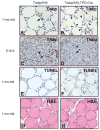
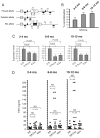
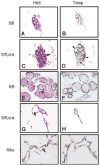
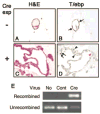
Thyroid-specific enhancer-binding protein (T/ebp)/Nkx2.1-null mouse thyroids degenerate by embryonic day (E) 12-13 through apoptosis whereas T/ebp/Nkx2.1-heterogyzgous mice exhibit hypothyroidism with elevated TSH levels. To understand the role of T/ebp/Nkx2.1 in the adult thyroid, a thyroid follicular cell-specific conditional knockout (KO) mouse line, T/ebp(fl/fl);TPO-Cre, was established that expresses Cre recombinase under the human thyroid peroxidase (TPO) gene promoter. These mice appeared to be healthy and exhibited loss of T/ebp/Nkx2.1 expression in many, but not all, thyroid follicular cells as determined by immunohistochemistry and real-time PCR, thus presenting a T/ebp-thyroid-conditional hypomorphic mice. Detailed analysis of the thyroids from T/ebp(fl/fl), T/ebp(fl/fl);TPO-Cre, and T/ebp(fl/ko) mice, where the latter mouse line is derived from crosses with the original T/ebp/Nkx2.1-heterozygous mice, revealed that T/ebp(fl/fl);TPO-Cre mice can be classified into two groups with different phenotypes: one having atrophic/degenerative thyroid follicles with frequent presence of adenomas and extremely high serum TSH levels, and the other having an altered thyroid structure with reduced numbers of extraordinary dilated follicles consisting of excessive numbers of follicular cells as compared with those usually found in the normal thyroid. The latter phenotype was also observed in aged T/ebp(fl/ko) mouse thyroids. In vitro three-dimensional thyroid primary cultures using thyroids from T/ebp(fl/fl);TPO-Cre, T/ebp(fl/ko), and T/ebp(fl/fl) mice, and the latter treated with recombinant adenovirus with and without Cre expression, demonstrated that only cells from T/ebp(fl/fl) mice without adeno-Cre treatment formed follicular structures. Taken together, these results suggest that T/ebp/Nkx2.1 is required for maintenance of the normal architecture and function of differentiated thyroids.
Figures


References
-
- Kaufman M, Bard J. The anatomical basis of mouse development. London: Academic Press; 1999.
-
- Manley NR, Capecchi MR. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Dev Biol. 1998;195:1–15. - PubMed
-
- Di Lauro R, De Felice M. Thyroid gland: anatomy and development. In: DeGroot L, Jameson J, editors. Endocrinology. Philadelphia: Saunders; 2001. pp. 1268–1278.
-
- Missero C, Cobellis G, De Felice M, Di Lauro R. Molecular events involved in differentiation of thyroid follicular cells. Mol Cell Endocrinol. 1998;140:37–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

