Extracellular matrix remodeling by dynamic strain in a three-dimensional tissue-engineered human airway wall model
- PMID: 16601241
- PMCID: PMC2643283
- DOI: 10.1165/rcmb.2005-0443OC
Extracellular matrix remodeling by dynamic strain in a three-dimensional tissue-engineered human airway wall model
Abstract
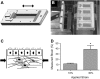
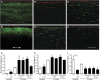
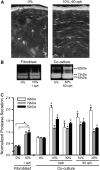
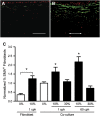
Airway wall remodeling is a hallmark of asthma, characterized by subepithelial thickening and extracellular matrix (ECM) remodeling. Mechanical stress due to hyperresponsive smooth muscle cells may contribute to this remodeling, but its relevance in a three-dimensional environment (where the ECM plays an important role in modulating stresses felt by cells) is unclear. To characterize the effects of dynamic compression in ECM remodeling in a physiologically relevant three-dimensional environment, a tissue-engineered human airway wall model with differentiated bronchial epithelial cells atop a collagen gel containing lung fibroblasts was used. Lateral compressive strain of 10 or 30% at 1 or 60 cycles per hour was applied using a novel straining device. ECM remodeling was assessed by immunohistochemistry and zymography. Dynamic strain, particularly at the lower magnitude, induced airway wall remodeling, as indicated by increased deposition of types III and IV collagen and increased secretion of matrix metalloproteinase-2 and -9. These changes paralleled increased myofibroblast differentiation and were fibroblast-dependent. Furthermore, the spatial pattern of type III collagen deposition correlated with that of myofibroblasts; both were concentrated near the epithelium and decreased diffusely away from the surface, indicating some epithelial control of the remodeling response. Thus, in a physiologically relevant three-dimensional model of the bronchial wall, dynamic compressive strain induced tissue remodeling that mimics many features of remodeling seen in asthma, in the absence of inflammation and dependent on epithelial-fibroblast signaling.
Figures

References
-
- James A. Airway remodeling in asthma. Curr Opin Pulm Med 2005;11: 1–6. - PubMed
-
- Homer RJ, Elias JA. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology (Bethesda) 2005;20:28–35. - PubMed
-
- Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol 2004;22:789–815. - PubMed
-
- McParland BE, Macklem PT, Pare PD. Airway wall remodeling: friend or foe? J Appl Physiol 2003;95:426–434. - PubMed
-
- Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 2001;164:S28–S38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

