Cockayne syndrome B protein regulates the transcriptional program after UV irradiation
- PMID: 16601682
- PMCID: PMC1456931
- DOI: 10.1038/sj.emboj.7601071
Cockayne syndrome B protein regulates the transcriptional program after UV irradiation
Abstract
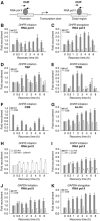
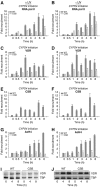
The phenotype of the human genetic disorder Cockayne syndrome (CS) is not only due to DNA repair defect but also (and perhaps essentially) to a severe transcription initiation defect. After UV irradiation, even undamaged genes are not transcribed in CSB cells. Indeed, neither RNA pol II nor the associated basal transcription factors are recruited to the promoters of the housekeeping genes, around of which histone H4 acetylation is also deficient. Transfection of CSB restores the recruitment process of RNA pol II. On the contrary, the p53-responsive genes do not require CSB and are transcribed in both wild-type and CSB cells upon DNA damage. Altogether, our data highlight the pivotal role of CSB in initiating the transcriptional program of certain genes after UV irradiation, and also may explain some of the complex traits of CS patients.
Figures



References
-
- Beerens N, Hoeijmakers JH, Kanaar R, Vermeulen W, Wyman C (2005) The CSB protein actively wraps DNA. J Biol Chem 280: 4722–4729 - PubMed
-
- Bird AW, Yu DY, Pray-Grant MG, Qiu Q, Harmon KE, Megee PC, Grant PA, Smith MM, Christman MF (2002) Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature 419: 411–415 - PubMed
-
- Bradsher J, Auriol J, Proietti de Santis L, Iben S, Vonesch JL, Grummt I, Egly JM (2002) CSB is a component of RNA pol I transcription. Mol Cell 10: 819–829 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

