Testing electrostatic complementarity in enzyme catalysis: hydrogen bonding in the ketosteroid isomerase oxyanion hole
- PMID: 16602823
- PMCID: PMC1413570
- DOI: 10.1371/journal.pbio.0040099
Testing electrostatic complementarity in enzyme catalysis: hydrogen bonding in the ketosteroid isomerase oxyanion hole
Abstract
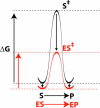
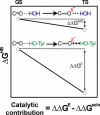
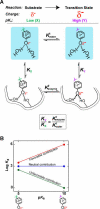
A longstanding proposal in enzymology is that enzymes are electrostatically and geometrically complementary to the transition states of the reactions they catalyze and that this complementarity contributes to catalysis. Experimental evaluation of this contribution, however, has been difficult. We have systematically dissected the potential contribution to catalysis from electrostatic complementarity in ketosteroid isomerase. Phenolates, analogs of the transition state and reaction intermediate, bind and accept two hydrogen bonds in an active site oxyanion hole. The binding of substituted phenolates of constant molecular shape but increasing pK(a) models the charge accumulation in the oxyanion hole during the enzymatic reaction. As charge localization increases, the NMR chemical shifts of protons involved in oxyanion hole hydrogen bonds increase by 0.50-0.76 ppm/pK(a) unit, suggesting a bond shortening of 0.02 A/pK(a) unit. Nevertheless, there is little change in binding affinity across a series of substituted phenolates (DeltaDeltaG = -0.2 kcal/mol/pK(a) unit). The small effect of increased charge localization on affinity occurs despite the shortening of the hydrogen bonds and a large favorable change in binding enthalpy (DeltaDeltaH = -2.0 kcal/mol/pK(a) unit). This shallow dependence of binding affinity suggests that electrostatic complementarity in the oxyanion hole makes at most a modest contribution to catalysis of 300-fold. We propose that geometrical complementarity between the oxyanion hole hydrogen-bond donors and the transition state oxyanion provides a significant catalytic contribution, and suggest that KSI, like other enzymes, achieves its catalytic prowess through a combination of modest contributions from several mechanisms rather than from a single dominant contribution.
Figures





Comment in
-
What governs enzyme activity? For one enzyme, charge contributes only weakly.PLoS Biol. 2006 Apr;4(4):e133. doi: 10.1371/journal.pbio.0040133. Epub 2006 Mar 28. PLoS Biol. 2006. PMID: 20076562 Free PMC article. No abstract available.
References
-
- Fersht AR. Catalysis, binding and enzyme-substrate complementarity. Proc R Soc Lond B Biol Sci. 1974;187:397–407. - PubMed
-
- Jencks WP. Binding energy, specificity, and enzymic catalysis: The Circe effect. Adv Enzymol Relat Areas Mol Biol. 1975;43:219–410. - PubMed
-
- Bruice TC. A view at the millennium: The efficiency of enzymatic catalysis. Acc Chem Res. 2002;35:139–148. - PubMed
-
- Bruice TC, Benkovic SJ. Bioorganic mechanisms. New York: W.A. Benjamin; 1966.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

