Inhibition of histone deacetylation in 293GPG packaging cell line improves the production of self-inactivating MLV-derived retroviral vectors
- PMID: 16603064
- PMCID: PMC1488828
- DOI: 10.1186/1743-422X-3-27
Inhibition of histone deacetylation in 293GPG packaging cell line improves the production of self-inactivating MLV-derived retroviral vectors
Abstract
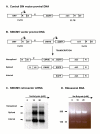
Background: Self-inactivating retroviral vectors (SIN) are often associated with very low titers. Promoter elements embedded within SIN designs may suppress transcription of packageable retroviral RNA which in turn results in titer reduction. We tested whether this dominant-negative effect involves histone acetylation state. We designed an MLV-derived SIN vector using the cytomegalovirus immediate early enhancer-promoter (CMVIE) as an embedded internal promoter (SINCMV) and transfected the pantropic 293GPG packaging cell line.
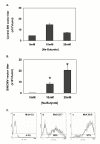
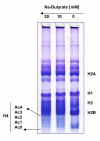
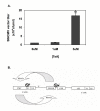
Results: The SINCMV retroviral producer had uniformly very low titers (approximately 10,000 infectious retroparticles per ml). Northern blot showed low levels of expression of retroviral mRNA in producer cells in particular that of packageable RNA transcript. Treatment of the producers with the histone deacetylase (HDAC) inhibitors sodium butyrate and trichostatin A reversed transcriptional suppression and resulted in an average 106.3 +/- 4.6 - fold (P = 0.002) and 15.5 +/- 1.3 - fold increase in titer (P = 0.008), respectively. A histone gel assay confirmed increased histone acetylation in treated producer cells.
Conclusion: These results show that SIN retrovectors incorporating strong internal promoters such as CMVIE, are susceptible to transcriptional silencing and that treatment of the producer cells with HDAC inhibitors can overcome this blockade suggesting that histone deacetylation is implicated in the mechanism of transcriptional suppression.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

