The role of steroids in follicular growth
- PMID: 16603089
- PMCID: PMC1459164
- DOI: 10.1186/1477-7827-4-16
The role of steroids in follicular growth
Abstract
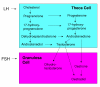
The steroidogenic pathway within the ovary gives rise to progestins, androgens and oestrogens, all of which act via specific nuclear receptors to regulate reproductive function and maintain fertility. The role of progestins in follicular growth and development is limited, its action confined largely to ovulation, although direct effects on granulosa cell function have been reported. Consistent with these findings, progesterone receptor knockout mice are infertile because they cannot ovulate. Androgens have been shown to promote early follicular growth, but also to impede follicular development by stimulating atresia and apoptosis. The inability of androgens to transduce a signal in mice lacking androgen receptors culminates in reduced fertility. Oestrogens are known to exert effects on granulosa cell growth and differentiation in association with gonadotrophins. Studies with oestrogen receptor knockouts and oestrogen depleted mice have shown us that oestrogen is essential for folliculogenesis beyond the antral stage and is necessary to maintain the female phenotype of ovarian somatic cells. In summary, the action of steroids within the ovary is based on the developmental status of the follicle. In the absence of any single sex steroid, ovarian function and subsequently fertility, are compromised.
Figures
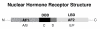
References
-
- White R, Parker MG. Molecular mechanisms of steroid hormone action. Endo Related Canc. 1998;5:1–14. doi: 10.1677/erc.0.0050001. - DOI
-
- Spelsberg TC, Steggles AW, Chytil F, O'Malley BW. Progesterone-binding components of chick oviduct. Exchange of progesterone-binding capacity from target to nontarget tissue chromatins. J Biol Chem. 1972;247:1368–1374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

