Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects
- PMID: 16603235
- PMCID: PMC2937188
- DOI: 10.1016/j.yfrne.2006.02.001
Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects
Abstract
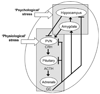
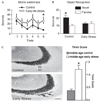
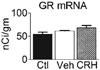
Whereas genetic factors contribute crucially to brain function, early-life events, including stress, exert long-lasting influence on neuronal function. Here, we focus on the hippocampus as the target of these early-life events because of its crucial role in learning and memory. Using a novel immature-rodent model, we describe the deleterious consequences of chronic early-life 'psychological' stress on hippocampus-dependent cognitive tasks. We review the cellular mechanisms involved and discuss the roles of stress-mediating molecules, including corticotropin releasing hormone, in the process by which stress impacts the structure and function of hippocampal neurons.
Figures

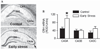
References
-
- Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. - PubMed
-
- Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur. J. Neurosci. 2003;17:1928–1934. - PubMed
-
- Altman J, Bayer SA. Prolonged sojourn of developing pyramidal cells in the intermediate zone of the hippocampus and their settling in the stratum pyramidale. J. Comp. Neurol. 1990;301:343–364. - PubMed
-
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J. Comp. Neurol. 1981;195:51–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

