Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system
- PMID: 16603603
- PMCID: PMC3481851
- DOI: 10.1096/fj.05-5134fje
Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system
Abstract
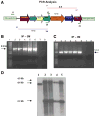
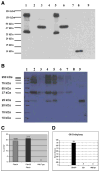
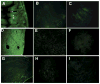
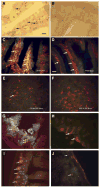
Oral delivery of biopharmaceutical proteins expressed in plant cells should reduce their cost of production, purification, processing, cold storage, transportation, and delivery. However, poor intestinal absorption of intact proteins is a major challenge. To overcome this limitation, we investigate here the concept of receptor-mediated oral delivery of chloroplast-expressed foreign proteins. Therefore, the transmucosal carrier cholera toxin B-subunit and green fluorescent protein (CTB-GFP), separated by a furin cleavage site, was expressed via the tobacco chloroplast genome. Polymerase chain reaction (PCR) and Southern blot analyses confirmed site-specific transgene integration and homoplasmy. Immunoblot analysis and ELISA confirmed expression of monomeric and pentameric forms of CTB-GFP, up to 21.3% of total soluble proteins. An in vitro furin cleavage assay confirmed integrity of the engineered furin cleavage site, and a GM1 binding assay confirmed the functionality of CTB-GFP pentamers. Following oral administration of CTB-GFP expressing leaf material to mice, GFP was observed in the mice intestinal mucosa, liver, and spleen in fluorescence and immunohistochemical studies, while CTB remained in the intestinal cell. This report of receptor-mediated oral delivery of a foreign protein into the circulatory system opens the door for low-cost production and delivery of human therapeutic proteins.
Figures


References
-
- Daniell H, Carmona-Sanchez O, Burns BE. Chloroplast derived antibodies, biopharmaceuticals and edible vaccines. In: Fischer R, Schillberg S, editors. Molecular Farming. WILEY-VCH Verlag Publishers; Weinheim, Germany: 2004. pp. 113–133.
-
- Petridis D, Sapidou E, Calandranis J. Computer aided process analysis and economic evaluation for biosynthetic human insulin production–a case study. Biotechnol Bioeng. 1995;48:529–541. - PubMed
-
- Giddings G, Allison G, Brooks D, Carter A. Transgenic plants as factories for biopharmaceuticals. Nat Biotechnol. 2000;18:1151–1155. - PubMed
-
- Arntzen C, Plotkin S, Dodet B. Plant-derived vaccines and antibodies: potential and limitations. Vaccine. 2005;23:1753–1756. - PubMed
-
- Mason HS, Warzecha H, Mor T, Arntzen C. Trends Mol Med. 2002;8:324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

