Spatiotemporal control of spindle midzone formation by PRC1 in human cells
- PMID: 16603632
- PMCID: PMC1458854
- DOI: 10.1073/pnas.0506926103
Spatiotemporal control of spindle midzone formation by PRC1 in human cells
Abstract
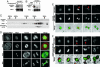
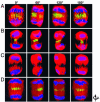
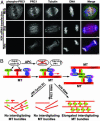
We have examined the role of PRC1, a midzone-associated, microtubule bundling, Cdk substrate protein, in regulating the spatiotemporal formation of the midzone in HeLa cells. Cdk-mediated phosphorylation of PRC1 in early mitosis holds PRC1 in an inactive monomeric state. During the metaphase-to-anaphase transition, PRC1 is dephosphorylated, promoting PRC1 oligomerization. Using time-lapse video microscopy, RNA interference, 3D immunofluorescence reconstruction imaging, and rescue experiments, we demonstrate that the dephosphorylated form of PRC1 is essential for bundling antiparallel, nonkinetochore, interdigitating microtubules to establish the midzone that is necessary for cytokinesis. Our results thus indicate that PRC1 is an essential factor in controlling the spatiotemporal formation of the midzone in human cells.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- D’Avino P. P., Savoian M. S., Glover D. M. J. Cell Sci. 2005;118:1549–1558. - PubMed
-
- McCollum D. Curr. Biol. 2004;14:R953–R955. - PubMed
-
- Mishima M., Kaitna S., Glotzer M. Dev. Cell. 2002;2:41–54. - PubMed
-
- Mishima M., Pavicic V., Gruneberg U., Nigg E. A., Glotzer M. Nature. 2004;430:908–913. - PubMed
-
- Bowerman B. Nature. 2004;430:840–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

