Decay of K103N mutants in cellular DNA and plasma RNA after single-dose nevirapine to reduce mother-to-child HIV transmission
- PMID: 16603851
- PMCID: PMC2841686
- DOI: 10.1097/01.aids.0000222071.60620.1d
Decay of K103N mutants in cellular DNA and plasma RNA after single-dose nevirapine to reduce mother-to-child HIV transmission
Abstract
Objectives: Single-dose nevirapine (sd-NVP) for prevention of mother-to-child HIV-1 transmission is associated with selection of resistant viral variants, particularly the Lysine (K) to Asparagine (N) mutation at codon 103 (K103N) of reverse transcriptase. As this may influence subsequent treatment responses, a better understanding of the dynamics of decay and persistence of this variant is needed.
Design and methods: We measured the frequency of K103N mutants among a cohort of HIV-1-infected pregnant women recruited at an out-patient clinic in Johannesburg, South Africa. Samples taken 6 weeks, 3, 7 and 12 months after delivery from 67 HIV-1-infected women who received sd-NVP during labor to prevent transmission were analyzed. Quantification of K103N mutants in maternal plasma viral RNA and cellular DNA was done using an allele-specific real-time polymerase chain reaction assay capable of detecting codons AAC and AAT if their frequency was > 0.002 of the total viral population.
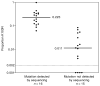
Results: Using the allele-specific assay, 87.1% (27/31) of RNA samples and 52.3% (23/44) of DNA samples collected 6 weeks after sd-NVP had detectable K103N variants. This declined to 65.4% (17/26), 38.9% (14/36), and 11.3% (6/53) in RNA at 3, 7 and 12 months respectively, and to 4.2% (2/48) in DNA at 12 months.
Conclusions: K103N resistant variants were present in almost all women at 6 weeks post-sd-NVP but declined rapidly over time. Resistant variants were detected less frequently in cellular DNA with persistence in this compartment by 12 months post-sd-NVP among only a minority.
Figures


References
-
- Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. - PubMed
-
- Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIV-NET 012 randomised trial. Lancet. 2003;362:859–868. - PubMed
-
- Jackson JB, Becker-Pergola G, Guay LA, Musoke P, Mracna M, Fowler MG, et al. Identification of the K103N resistance mutation in Ugandan women receiving nevirapine to prevent HIV-1 vertical transmission. AIDS. 2000;14:F111–F115. - PubMed
-
- Martinson N, Morris L, Gray G, Moodley D, Lupondwana P, Chezzi C. HIV resistance and transmission following single-dose nevirapine in a PMTCT cohort. Eleventh Conference on Retroviruses and Opportunistic Infections; San Francisco. February 2004; [abstract 38]
-
- Eshleman SH, Mracna M, Guay LA, Deseyve M, Cunningham S, Mirochnick M, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15 :1951–1957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

