Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells
- PMID: 16604075
- PMCID: PMC3725256
- DOI: 10.1038/ni1327
Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells
Abstract
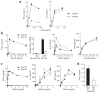
The observation that the T-bet transcription factor allows tissue-specific upregulation of intracellular osteopontin (Opn-i) in plasmacytoid dendritic cells (pDCs) suggests that Opn might contribute to the expression of interferon-alpha (IFN-alpha) in those cells. Here we show that Opn deficiency substantially reduced Toll-like receptor 9 (TLR9)-dependent IFN-alpha responses but spared expression of transcription factor NF-kappaB-dependent proinflammatory cytokines. Shortly after TLR9 engagement, colocalization of Opn-i and the adaptor molecule MyD88 was associated with induction of transcription factor IRF7-dependent IFN-alpha gene expression, whereas deficient expression of Opn-i was associated with defective nuclear translocation of IRF7 in pDCs. The importance of the Opn-IFN-alpha pathway was emphasized by its essential involvement in cross-presentation in vitro and in anti-herpes simplex virus 1 IFN-alpha response in vivo. The finding that Opn-i selectively coupled TLR9 signaling to expression of IFN-alpha but not to that of other proinflammatory cytokines provides new molecular insight into the biology of pDCs.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
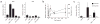
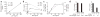
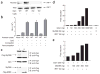
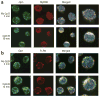

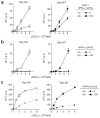
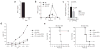
Comment in
-
Opn: key regulator of pDC interferon production.Nat Immunol. 2006 May;7(5):441-3. doi: 10.1038/ni0506-441. Nat Immunol. 2006. PMID: 16622428 No abstract available.
References
-
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. - PubMed
-
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. - PubMed
-
- Krug A, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. - PubMed
-
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. - PubMed
-
- Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

