Activation of Peptide ions by blackbody radiation: factors that lead to dissociation kinetics in the rapid energy exchange limit
- PMID: 16604162
- PMCID: PMC1434517
- DOI: 10.1021/jp9722418
Activation of Peptide ions by blackbody radiation: factors that lead to dissociation kinetics in the rapid energy exchange limit
Abstract
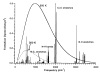
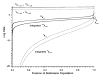
Unimolecular rate constants for blackbody infrared radiative dissociation (BIRD) were calculated for the model protonated peptide (AlaGly)(n) (n = 2-32) using a variety of dissociation parameters. Combinations of dissociation threshold energies ranging from 0.8 to 1.7 eV and transition entropies corresponding to Arrhenius preexponential factors ranging from very "tight" (A(infinity) = 10(9.9) s(-1)) to "loose" (A(infinity) = 10(16.8) s(-1)) were selected to represent dissociation parameters within the experimental temperature range (300-520 K) and kinetic window (k(uni) = 0.001-0.20 s(-1)) typically used in the BIRD experiment. Arrhenius parameters were determined from the temperature dependence of these values and compared to those in the rapid energy exchange (REX) limit. In this limit, the internal energy of a population of ions is given by a Boltzmann distribution, and kinetics are the same as those in the traditional high-pressure limit. For a dissociation process to be in this limit, the rate of photon exchange between an ion and the vacuum chamber walls must be significantly greater than the dissociation rate. Kinetics rapidly approach the REX limit either as the molecular size or threshold dissociation energy increases or as the transition-state entropy or experimental temperature decreases. Under typical experimental conditions, peptide ions larger than 1.6 kDa should be in the REX limit. Smaller ions may also be in the REX limit depending on the value of the threshold dissociation energy and transition-state entropy. Either modeling or information about the dissociation mechanism must be known in order to confirm REX limit kinetics for these smaller ions. Three principal factors that lead to the size dependence of REX limit kinetics are identified. With increasing molecular size, rates of radiative absorption and emission increase, internal energy distributions become relatively narrower, and the microcanonical dissociation rate constants increase more slowly over the energy distribution of ions. Guidelines established here should make BIRD an even more reliable method to obtain information about dissociation energetics and mechanisms for intermediate size molecules.
Figures



Similar articles
-
Dissociation energetics and mechanisms of leucine enkephalin (M + H)+ and (2M + X)+ ions (X = H, Li, Na, K, and Rb) measured by blackbody infrared radiative dissociation.J Am Soc Mass Spectrom. 1997 Aug;8(8):771-80. doi: 10.1016/S1044-0305(97)84129-3. J Am Soc Mass Spectrom. 1997. PMID: 16554908 Free PMC article.
-
Effects of charge state on fragmentation pathways, dynamics, and activation energies of ubiquitin ions measured by blackbody infrared radiative dissociation.Anal Chem. 1997 Mar 15;69(6):1119-26. doi: 10.1021/ac960804q. Anal Chem. 1997. PMID: 9075403 Free PMC article.
-
Blackbody infrared radiative dissociation of bradykinin and its analogues: energetics, dynamics, and evidence for salt-bridge structures in the gas phase.J Am Chem Soc. 1996 Jul 31;118(30):7178-89. doi: 10.1021/ja9609157. J Am Chem Soc. 1996. PMID: 16525512 Free PMC article.
-
Collisional activation of peptide ions in FT-ICR mass spectrometry.Mass Spectrom Rev. 2003 May-Jun;22(3):158-81. doi: 10.1002/mas.10041. Mass Spectrom Rev. 2003. PMID: 12838543 Review.
-
Ion activation methods for tandem mass spectrometry.J Mass Spectrom. 2004 Oct;39(10):1091-112. doi: 10.1002/jms.703. J Mass Spectrom. 2004. PMID: 15481084 Review.
Cited by
-
Collision-induced dissociation of bradykinin ions in the interface region of an ESI-MS.J Am Soc Mass Spectrom. 2001 Jul;12(7):772-9. doi: 10.1016/S1044-0305(01)00266-5. J Am Soc Mass Spectrom. 2001. PMID: 11444598
-
Effects of electron kinetic energy and ion-electron inelastic collisions in electron capture dissociation measured using ion nanocalorimetry.J Am Soc Mass Spectrom. 2008 Jun;19(6):772-9. doi: 10.1016/j.jasms.2008.02.010. Epub 2008 Mar 5. J Am Soc Mass Spectrom. 2008. PMID: 18372190 Free PMC article.
-
Electron capture by a hydrated gaseous peptide: effects of water on fragmentation and molecular survival.J Am Chem Soc. 2008 Sep 24;130(38):12680-9. doi: 10.1021/ja8022434. Epub 2008 Aug 30. J Am Chem Soc. 2008. PMID: 18761457 Free PMC article.
-
Relative stability of peptide sequence ions generated by tandem mass spectrometry.J Am Soc Mass Spectrom. 2012 Apr;23(4):644-54. doi: 10.1007/s13361-012-0357-3. Epub 2012 Feb 22. J Am Soc Mass Spectrom. 2012. PMID: 22354685
-
Binding energies of water to lithiated valine: formation of solution-phase structure in vacuo.J Am Soc Mass Spectrom. 2004 Jul;15(7):1014-24. doi: 10.1016/j.jasms.2004.04.001. J Am Soc Mass Spectrom. 2004. PMID: 15234361
References
-
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64. - PubMed
-
- Smith RD, Loo JA, Ogorzalek Loo RR, Busman M, Udseth HR. Mass Spectrom Rev. 1991;10:359.
-
- Hillenkamp F, Karas M, Beavis RC, Chait BT. Anal Chem. 1991;63:1193A. - PubMed
-
- Alexander AJ, Boyd AK. Int J Mass Spectrom Ion Processes. 1989;90:211.
-
- Marzluff EM, Campbell S, Rodgers MT, Beauchamp JL. J Am Chem Soc. 1994;116:7787–7796.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
