Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase
- PMID: 16604191
- PMCID: PMC1430359
- DOI: 10.1172/JCI27607
Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase
Abstract
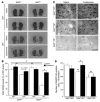
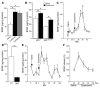
There is no treatment for the neurodegenerative disorder Huntington disease (HD). Cystamine is a candidate drug; however, the mechanisms by which it operates remain unclear. We show here that cystamine increases levels of the heat shock DnaJ-containing protein 1b (HSJ1b) that are low in HD patients. HSJ1b inhibits polyQ-huntingtin-induced death of striatal neurons and neuronal dysfunction in Caenorhabditis elegans. This neuroprotective effect involves stimulation of the secretory pathway through formation of clathrin-coated vesicles containing brain-derived neurotrophic factor (BDNF). Cystamine increases BDNF secretion from the Golgi region that is blocked by reducing HSJ1b levels or by overexpressing transglutaminase. We demonstrate that cysteamine, the FDA-approved reduced form of cystamine, is neuroprotective in HD mice by increasing BDNF levels in brain. Finally, cysteamine increases serum levels of BDNF in mouse and primate models of HD. Therefore, cysteamine is a potential treatment for HD, and serum BDNF levels can be used as a biomarker for drug efficacy.
Figures


Similar articles
-
Cystamine increases L-cysteine levels in Huntington's disease transgenic mouse brain and in a PC12 model of polyglutamine aggregation.J Neurochem. 2004 Oct;91(2):413-22. doi: 10.1111/j.1471-4159.2004.02726.x. J Neurochem. 2004. PMID: 15447674
-
Cystamine treatment is neuroprotective in the YAC128 mouse model of Huntington disease.J Neurochem. 2005 Oct;95(1):210-20. doi: 10.1111/j.1471-4159.2005.03357.x. J Neurochem. 2005. PMID: 16181425
-
Cystamine metabolism and brain transport properties: clinical implications for neurodegenerative diseases.J Neurochem. 2010 Sep;114(6):1651-8. doi: 10.1111/j.1471-4159.2010.06874.x. Epub 2010 Aug 19. J Neurochem. 2010. PMID: 20569301
-
Potential of cystamine and cysteamine in the treatment of neurodegenerative diseases.Prog Neuropsychopharmacol Biol Psychiatry. 2011 Mar 30;35(2):380-9. doi: 10.1016/j.pnpbp.2010.11.023. Epub 2010 Nov 24. Prog Neuropsychopharmacol Biol Psychiatry. 2011. PMID: 21111020 Review.
-
Cystamine and cysteamine as inhibitors of transglutaminase activity in vivo.Biosci Rep. 2018 Sep 5;38(5):BSR20180691. doi: 10.1042/BSR20180691. Print 2018 Oct 31. Biosci Rep. 2018. PMID: 30054429 Free PMC article. Review.
Cited by
-
Transglutaminase inhibition protects against oxidative stress-induced neuronal death downstream of pathological ERK activation.J Neurosci. 2012 May 9;32(19):6561-9. doi: 10.1523/JNEUROSCI.3353-11.2012. J Neurosci. 2012. PMID: 22573678 Free PMC article.
-
Cystamine attenuated behavioral deficiency via increasing the expression of BDNF and activating PI3K/Akt signaling in 2,5-hexanedione intoxicated rats.Toxicol Res (Camb). 2016 Dec 12;6(2):199-204. doi: 10.1039/c6tx00409a. eCollection 2017 Mar 1. Toxicol Res (Camb). 2016. PMID: 30090490 Free PMC article.
-
Genetics and treatment options for recurrent acute and chronic pancreatitis.Curr Treat Options Gastroenterol. 2014 Sep;12(3):359-71. doi: 10.1007/s11938-014-0022-y. Curr Treat Options Gastroenterol. 2014. PMID: 24954874 Free PMC article.
-
Cysteamine alleviates early brain injury via reducing oxidative stress and apoptosis in a rat experimental subarachnoid hemorrhage model.Cell Mol Neurobiol. 2015 May;35(4):543-53. doi: 10.1007/s10571-014-0150-x. Epub 2014 Dec 20. Cell Mol Neurobiol. 2015. PMID: 25527033 Free PMC article.
-
Inhibition of transglutaminase 2 mitigates transcriptional dysregulation in models of Huntington disease.EMBO Mol Med. 2010 Sep;2(9):349-70. doi: 10.1002/emmm.201000084. EMBO Mol Med. 2010. PMID: 20665636 Free PMC article.
References
-
- MacDonald M.E., Gines S., Gusella J.F., Wheeler V.C. Huntington’s disease. Neuromolecular Med. 2003;4:7–20. - PubMed
-
- Melino G., Piacentini M. ‘Tissue’ transglutaminase in cell death: a downstream or a multifunctional upstream effector? FEBS Lett. 1998;430:59–63. - PubMed
-
- Lesort M., Tucholski J., Miller M.L., Johnson G.V. Tissue transglutaminase: a possible role in neurodegenerative diseases. Prog. Neurobiol. 2000;61:439–463. - PubMed
-
- Green H. Human genetic diseases due to codon reiteration: relationship to an evolutionary mechanism. Cell. 1993;74:955–956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

