"Viral déjà vu" elicits organ-specific immune disease independent of reactivity to self
- PMID: 16604192
- PMCID: PMC1430358
- DOI: 10.1172/JCI27372
"Viral déjà vu" elicits organ-specific immune disease independent of reactivity to self
Abstract
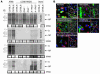
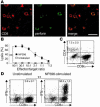
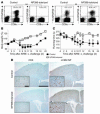
Autoimmune diseases are often precipitated by viral infections. Yet our current understanding fails to explain how viruses trigger organ-specific autoimmunity despite thymic tolerance extending to many non-lymphohematopoietic self antigens. Additionally, a key epidemiological finding needs to be explained: In genetically susceptible individuals, early childhood infections seem to predispose them to multiple sclerosis (MS) or type 1 diabetes years or even decades before clinical onset. In the present work, we show that the innate immune system of neonatal mice was sufficient to eliminate an attenuated lymphocytic choriomeningitis virus (LCMV) from most tissues except for the CNS, where the virus persisted in neurons (predisposing virus). Virus-specific cytotoxic T cells (CTLs) were neither deleted nor sufficiently primed to cause disease, but they were efficiently triggered in adulthood upon WT LCMV infection (precipitating virus). This defined sequence of viral infections caused severe CNS inflammation that was histomorphologically reminiscent of rasmussen encephalitis, a fatal human autoimmune disease. Yet disease in mice was mediated by antiviral CTLs targeting an epitope shared by the precipitating virus and the predisposing virus persisting in neurons (déjà vu). Thus the concept of "viral déjà vu" demonstrates how 2 related but independently encountered viral infections can cause organ-specific immune disease without molecular mimicry of self and without breaking self tolerance.
Figures

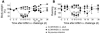

Similar articles
-
Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease.J Exp Med. 1996 Dec 1;184(6):2371-84. doi: 10.1084/jem.184.6.2371. J Exp Med. 1996. PMID: 8976191 Free PMC article.
-
Multiple sclerosis and virus induced immune responses: autoimmunity can be primed by molecular mimicry and augmented by bystander activation.Autoimmunity. 2006 Feb;39(1):9-19. doi: 10.1080/08916930500484799. Autoimmunity. 2006. PMID: 16455578 Review.
-
Using transgenic mouse models to dissect the pathogenesis of virus-induced autoimmune disorders of the islets of Langerhans and the central nervous system.Immunol Rev. 1996 Aug;152:111-43. doi: 10.1111/j.1600-065x.1996.tb00913.x. Immunol Rev. 1996. PMID: 8930670 Review.
-
Viral hyperinfection of the central nervous system and high mortality after hematopoietic stem cell transplantation for treatment of Theiler's murine encephalomyelitis virus-induced demyelinating disease.Blood. 1999 Oct 15;94(8):2915-22. Blood. 1999. PMID: 10515897
-
T and B cell tolerance and responses to viral antigens in transgenic mice: implications for the pathogenesis of autoimmune versus immunopathological disease.Immunol Rev. 1991 Aug;122:133-71. doi: 10.1111/j.1600-065x.1991.tb00601.x. Immunol Rev. 1991. PMID: 1937540 Review.
Cited by
-
Regulation of immune response and inflammatory reactions against viral infection by VCAM-1.J Virol. 2008 Mar;82(6):2952-65. doi: 10.1128/JVI.02191-07. Epub 2008 Jan 23. J Virol. 2008. PMID: 18216105 Free PMC article.
-
Evaluating epigenetic landmarks in the brain of multiple sclerosis patients: a contribution to the current debate on disease pathogenesis.Prog Neurobiol. 2008 Dec 11;86(4):368-78. doi: 10.1016/j.pneurobio.2008.09.012. Epub 2008 Sep 26. Prog Neurobiol. 2008. PMID: 18930111 Free PMC article. Review.
-
Detection of EBV and HHV6 in the Brain Tissue of Patients with Rasmussen's Encephalitis.Virol Sin. 2018 Oct;33(5):402-409. doi: 10.1007/s12250-018-0063-9. Epub 2018 Oct 29. Virol Sin. 2018. PMID: 30374827 Free PMC article.
-
The neuropathobiology of multiple sclerosis.Nat Rev Neurosci. 2024 Jul;25(7):493-513. doi: 10.1038/s41583-024-00823-z. Epub 2024 May 24. Nat Rev Neurosci. 2024. PMID: 38789516 Review.
-
Expression of human cytomegalovirus components in the brain tissues of patients with Rasmussen's encephalitis.Virol Sin. 2017 Apr;32(2):115-121. doi: 10.1007/s12250-016-3917-z. Epub 2017 Mar 2. Virol Sin. 2017. PMID: 28303446 Free PMC article.
References
-
- Marie P. Sclerose en plaque et maladie infecteuses. Prog. Med. 1884;12:287–289.
-
- Ebers G.C., et al. A population-based study of multiple sclerosis in twins. N. Engl. J. Med. 1986;315:1638–1642. - PubMed
-
- Dyment D.A., Ebers G.C., Sadovnick A.D. Genetics of multiple sclerosis. Lancet Neurol. 2004;3:104–110. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

