Cross-correlation of instantaneous phase increments in pressure-flow fluctuations: applications to cerebral autoregulation
- PMID: 16605566
- PMCID: PMC2140229
- DOI: 10.1103/PhysRevE.73.031915
Cross-correlation of instantaneous phase increments in pressure-flow fluctuations: applications to cerebral autoregulation
Abstract
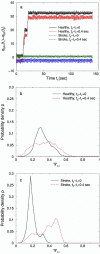
We investigate the relationship between the blood flow velocities (BFV) in the middle cerebral arteries and beat-to-beat blood pressure (BP) recorded from a finger in healthy and post-stroke subjects during the quasisteady state after perturbation for four different physiologic conditions: supine rest, head-up tilt, hyperventilation, and CO2 rebreathing in upright position. To evaluate whether instantaneous BP changes in the steady state are coupled with instantaneous changes in the BFV, we compare dynamical patterns in the instantaneous phases of these signals, obtained from the Hilbert transform, as a function of time. We find that in post-stroke subjects the instantaneous phase increments of BP and BFV exhibit well-pronounced patterns that remain stable in time for all four physiologic conditions, while in healthy subjects these patterns are different, less pronounced, and more variable. We propose an approach based on the cross-correlation of the instantaneous phase increments to quantify the coupling between BP and BFV signals. We find that the maximum correlation strength is different for the two groups and for the different conditions. For healthy subjects the amplitude of the cross-correlation between the instantaneous phase increments of BP and BFV is small and attenuates within 3-5 heartbeats. In contrast, for post-stroke subjects, this amplitude is significantly larger and cross-correlations persist up to 20 heartbeats. Further, we show that the instantaneous phase increments of BP and BFV are cross-correlated even within a single heartbeat cycle. We compare the results of our approach with three complementary methods: direct BP-BFV cross-correlation, transfer function analysis, and phase synchronization analysis. Our findings provide insight into the mechanism of cerebral vascular control in healthy subjects, suggesting that this control mechanism may involve rapid adjustments (within a heartbeat) of the cerebral vessels, so that BFV remains steady in response to changes in peripheral BP.
Figures




Similar articles
-
Nonlinear assessment of cerebral autoregulation from spontaneous blood pressure and cerebral blood flow fluctuations.Cardiovasc Eng. 2008 Mar;8(1):60-71. doi: 10.1007/s10558-007-9045-5. Cardiovasc Eng. 2008. PMID: 18080758 Free PMC article.
-
Multimodal pressure-flow method to assess dynamics of cerebral autoregulation in stroke and hypertension.Biomed Eng Online. 2004 Oct 25;3(1):39. doi: 10.1186/1475-925X-3-39. Biomed Eng Online. 2004. PMID: 15504235 Free PMC article.
-
Autoregulation of cerebral blood flow in orthostatic hypotension.Stroke. 1998 Jan;29(1):104-11. doi: 10.1161/01.str.29.1.104. Stroke. 1998. PMID: 9445337
-
Altered cerebral vasoregulation in hypertension and stroke.Neurology. 2003 May 27;60(10):1657-63. doi: 10.1212/01.wnl.0000068023.14587.06. Neurology. 2003. PMID: 12771258
-
Cerebrovascular regulation in the postural orthostatic tachycardia syndrome (POTS).Am J Med Sci. 1999 Feb;317(2):124-33. doi: 10.1097/00000441-199902000-00007. Am J Med Sci. 1999. PMID: 10037116 Review.
Cited by
-
Increased phase synchronization and decreased cerebral autoregulation during fainting in the young.Am J Physiol Heart Circ Physiol. 2009 Dec;297(6):H2084-95. doi: 10.1152/ajpheart.00705.2009. Epub 2009 Oct 9. Am J Physiol Heart Circ Physiol. 2009. PMID: 19820196 Free PMC article.
-
Complexity synchronization analysis of neurophysiological data: Theory and methods.Front Netw Physiol. 2025 May 14;5:1570530. doi: 10.3389/fnetp.2025.1570530. eCollection 2025. Front Netw Physiol. 2025. PMID: 40438270 Free PMC article.
-
How stimulation frequency and intensity impact on the long-lasting effects of coordinated reset stimulation.PLoS Comput Biol. 2018 May 10;14(5):e1006113. doi: 10.1371/journal.pcbi.1006113. eCollection 2018 May. PLoS Comput Biol. 2018. PMID: 29746458 Free PMC article.
-
Multivariate linear time-series modeling and prediction of cerebral physiologic signals: review of statistical models and implications for human signal analytics.Front Netw Physiol. 2025 Apr 16;5:1551043. doi: 10.3389/fnetp.2025.1551043. eCollection 2025. Front Netw Physiol. 2025. PMID: 40308555 Free PMC article. Review.
-
A Brief Review of Chimera State in Empirical Brain Networks.Front Physiol. 2020 Jun 30;11:724. doi: 10.3389/fphys.2020.00724. eCollection 2020. Front Physiol. 2020. PMID: 32714208 Free PMC article. Review.
References
-
- Lassen NA. Physiol. Rev. 1959;39:183. - PubMed
-
- Narayanan K, Collins JJ, Hamner J, Mukai S, Lipsitz LA. Am. J. Physiol. Regulatory Integrative Comp. Physiol. 2001;281:R716. - PubMed
-
- Panerai RB. Physiol. Meas. 1998;19:305. - PubMed
-
- Schwarz S, Georgiadis D, Aschoff A, Schwab S. Stroke. 2002;33:497. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
