Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation
- PMID: 16606348
- PMCID: PMC1533314
- DOI: 10.1042/BJ20060125
Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation
Abstract
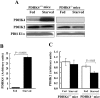
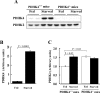
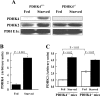
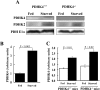
The PDC (pyruvate dehydrogenase complex) is strongly inhibited by phosphorylation during starvation to conserve substrates for gluconeogenesis. The role of PDHK4 (pyruvate dehydrogenase kinase isoenzyme 4) in regulation of PDC by this mechanism was investigated with PDHK4-/- mice (homozygous PDHK4 knockout mice). Starvation lowers blood glucose more in mice lacking PDHK4 than in wild-type mice. The activity state of PDC (percentage dephosphorylated and active) is greater in kidney, gastrocnemius muscle, diaphragm and heart but not in the liver of starved PDHK4-/- mice. Intermediates of the gluconeogenic pathway are lower in concentration in the liver of starved PDHK4-/- mice, consistent with a lower rate of gluconeogenesis due to a substrate supply limitation. The concentration of gluconeogenic substrates is lower in the blood of starved PDHK4-/- mice, consistent with reduced formation in peripheral tissues. Isolated diaphragms from starved PDHK4-/- mice accumulate less lactate and pyruvate because of a faster rate of pyruvate oxidation and a reduced rate of glycolysis. BCAAs (branched chain amino acids) are higher in the blood in starved PDHK4-/- mice, consistent with lower blood alanine levels and the importance of BCAAs as a source of amino groups for alanine formation. Non-esterified fatty acids are also elevated more in the blood of starved PDHK4-/- mice, consistent with lower rates of fatty acid oxidation due to increased rates of glucose and pyruvate oxidation due to greater PDC activity. Up-regulation of PDHK4 in tissues other than the liver is clearly important during starvation for regulation of PDC activity and glucose homoeostasis.
Figures




References
-
- Holness M. J., Sugden M. C. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem. Soc. Trans. 2003;31:1143–1151. - PubMed
-
- Roche T. E., Baker J. C., Yan X., Hiromasa Y., Gong X., Peng T., Dong J., Turkan A., Kasten S. A. Distinct regulatory properties of pyruvate dehydrogenase kinase and phosphatase isoforms. Prog. Nucleic Acid Res. Mol. Biol. 2001;70:33–75. - PubMed
-
- Behal R. H., Buxton D. B., Robertson J. G., Olson M. S. Regulation of the pyruvate dehydrogenase multienzyme complex. Annu. Rev. Nutr. 1993;13:497–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

