Differential activation of inflammatory pathways in A549 type II pneumocytes by Streptococcus pneumoniae strains with different adherence properties
- PMID: 16606470
- PMCID: PMC1481607
- DOI: 10.1186/1471-2334-6-71
Differential activation of inflammatory pathways in A549 type II pneumocytes by Streptococcus pneumoniae strains with different adherence properties
Abstract
Background: Adherence of Streptococcus pneumoniae bacteria to lung cells is a first step in the progression from asymptomatic carriage to pneumonia. Adherence abilities vary widely among S. pneumoniae patient isolates. In this study, the binding properties of S. pneumoniae isolates and the effects of binding on activation of the Nuclear Factor-Kappa-B (NFkappaB) pathway and cytokine secretion by type II pneumocytes were measured.
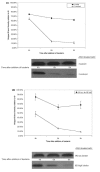
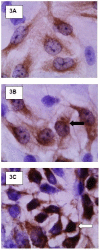
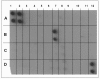
Methods: Mechanisms of high- and low-binding S. pneumoniae adherence to A549 cells were investigated by blocking putative receptors on bacteria and host cells with antibody and by eluting choline-binding proteins off of bacterial surfaces. NFkappaB activation was measured by western blot and immunocytochemistry and cytokine secretion was detected by a protein array.
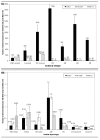
Results: This study shows that S. pneumoniae isolates from pneumonia patients (n = 298) can vary by as much as 1000-fold in their ability to bind to human lung epithelial cells. This difference resulted in differential activation of the NFkappaB pathway. High-, but not low-binding S. pneumoniae used Choline-binding protein A (CbpA) to bind to complement component C3 on epithelial cell surfaces. Interleukin-8 (IL-8) was the only cytokine secreted by cells treated with either low- or high-binding S. pneumoniae.
Conclusion: These results indicate that S. pneumoniae clinical isolates are not homogeneous in their interaction with host epithelial cells. The differential activation of host cells by high- and low-binding S. pneumoniae strains could have implications for the treatment of pneumococcal pneumonia and for vaccine development.
Figures
Similar articles
-
C3 as substrate for adhesion of Streptococcus pneumoniae.J Infect Dis. 2000 Aug;182(2):497-508. doi: 10.1086/315722. Epub 2000 Jul 19. J Infect Dis. 2000. PMID: 10915081
-
Preceding human metapneumovirus infection increases adherence of Streptococcus pneumoniae and severity of murine pneumococcal pneumonia.J Microbiol Immunol Infect. 2016 Apr;49(2):216-24. doi: 10.1016/j.jmii.2014.04.008. Epub 2014 Jun 13. J Microbiol Immunol Infect. 2016. PMID: 24931548
-
Streptococcus pneumoniae-Induced Oxidative Stress in Lung Epithelial Cells Depends on Pneumococcal Autolysis and Is Reversible by Resveratrol.J Infect Dis. 2015 Jun 1;211(11):1822-30. doi: 10.1093/infdis/jiu806. Epub 2014 Dec 15. J Infect Dis. 2015. PMID: 25512625
-
PAf receptor anchors Streptococcus pneumoniae to activated human endothelial cells.Adv Exp Med Biol. 1996;416:89-94. doi: 10.1007/978-1-4899-0179-8_16. Adv Exp Med Biol. 1996. PMID: 9131132 Review.
-
Pathogenesis of pneumococcal infection.N Engl J Med. 1995 May 11;332(19):1280-4. doi: 10.1056/NEJM199505113321907. N Engl J Med. 1995. PMID: 7708073 Review. No abstract available.
Cited by
-
Roles of virulence genes (PsaA and CpsA) on the invasion of Streptococcus pneumoniae into blood system.Eur J Med Res. 2013 May 17;18(1):14. doi: 10.1186/2047-783X-18-14. Eur J Med Res. 2013. PMID: 23683724 Free PMC article.
-
Impact of different Streptococcus pneumoniae on the secretion of interleukin and adhesin from THP-1 monocytes.J Clin Lab Anal. 2019 Sep;33(7):e22927. doi: 10.1002/jcla.22927. Epub 2019 Jun 23. J Clin Lab Anal. 2019. PMID: 31231868 Free PMC article.
-
The histone demethylase KDM6B fine-tunes the host response to Streptococcus pneumoniae.Nat Microbiol. 2021 Feb;6(2):257-269. doi: 10.1038/s41564-020-00805-8. Epub 2020 Dec 21. Nat Microbiol. 2021. PMID: 33349663
-
Role of Pht proteins in attachment of Streptococcus pneumoniae to respiratory epithelial cells.Infect Immun. 2014 Apr;82(4):1683-91. doi: 10.1128/IAI.00699-13. Epub 2014 Feb 3. Infect Immun. 2014. PMID: 24491577 Free PMC article.
-
Factor H binding to PspC of Streptococcus pneumoniae increases adherence to human cell lines in vitro and enhances invasion of mouse lungs in vivo.Infect Immun. 2007 Aug;75(8):4082-7. doi: 10.1128/IAI.00474-07. Epub 2007 Jun 11. Infect Immun. 2007. PMID: 17562771 Free PMC article.
References
-
- Austrian R. Some aspects of the pneumococcal carrier state. J Antimicrob Chemother. 1986;18 Suppl A:35–45. - PubMed
-
- Hendley JO, Sande MA, Stewart PM, Gwaltney JMJ. Spread of Streptococcus pneumoniae in families. I. Carriage rates and distribution of types. J Infect Dis. 1975;132:55–61. - PubMed
-
- Cockeran R, Anderson R, Feldman C. Pneumolysin as a vaccine and drug target in the prevention and treatment of invasive pneumococcal disease. Arch Immunol Ther Exp (Warsz ) 2005;53:189–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

