Evidence for discrete stages of human natural killer cell differentiation in vivo
- PMID: 16606675
- PMCID: PMC2118285
- DOI: 10.1084/jem.20052507
Evidence for discrete stages of human natural killer cell differentiation in vivo
Abstract
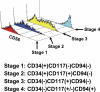
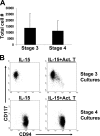
Human natural killer (NK) cells originate from CD34(+) hematopoietic progenitor cells, but the discrete stages of NK cell differentiation in vivo have not been elucidated. We identify and functionally characterize, from human lymph nodes and tonsils, four NK cell developmental intermediates spanning the continuum of differentiation from a CD34(+) NK cell progenitor to a functionally mature NK cell. Analyses of each intermediate stage for CD34, CD117, and CD94 cell surface expression, lineage differentiation potentials, capacity for cytokine production and natural cytotoxicity, and ETS-1, GATA-3, and T-BET expression provide evidence for a new model of human NK cell differentiation in secondary lymphoid tissues.
Figures







References
-
- Colucci, F., M.A. Caligiuri, and J.P. Di Santo. 2003. What does it take to make a natural killer? Nat. Rev. Immunol. 3:413–425. - PubMed
-
- Yokoyama, W.M., S. Kim, and A.R. French. 2004. The dynamic life of natural killer cells. Annu. Rev. Immunol. 22:405–429. - PubMed
-
- Mrozek, E., P. Anderson, and M.A. Caligiuri. 1996. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 87:2632–2640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

