Coupling modes and stoichiometry of Cl-/HCO3- exchange by slc26a3 and slc26a6
- PMID: 16606687
- PMCID: PMC2151520
- DOI: 10.1085/jgp.200509392
Coupling modes and stoichiometry of Cl-/HCO3- exchange by slc26a3 and slc26a6
Abstract
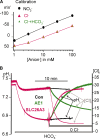
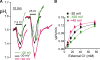
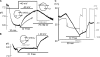
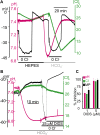
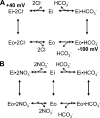
The SLC26 transporters are a family of mostly luminal Cl- and HCO3- transporters. The transport mechanism and the Cl-/HCO3- stoichiometry are not known for any member of the family. To address these questions, we simultaneously measured the HCO3- and Cl- fluxes and the current or membrane potential of slc26a3 and slc26a6 expressed in Xenopus laevis oocytes and the current of the transporters expressed in human embryonic kidney 293 cells. slc26a3 mediates a coupled 2Cl-/1HCO3- exchanger. The membrane potential modulated the apparent affinity for extracellular Cl- of Cl-/HCO3- exchange by slc26a3. Interestingly, the replacement of Cl- with NO3- or SCN- uncoupled the transport, with large NO3- and SCN- currents and low HCO3- transport. An apparent uncoupled current was also developed during the incubation of slc26a3-expressing oocytes in HCO3--buffered Cl--free media. These findings were used to develop a turnover cycle for Cl- and HCO3- transport by slc26a3. Cl- and HCO3- flux measurements revealed that slc26a6 mediates a 1Cl-/2HCO3- exchange. Accordingly, holding the membrane potential at 40 and -100 mV accelerated and inhibited, respectively, Cl--mediated HCO3- influx, and holding the membrane potential at -100 mV increased HCO3--mediated Cl- influx. These findings indicate that slc26a6 functions as a coupled 1Cl-/2HCO3- exchanger. The significance of isoform-specific Cl- and HCO3- transport stoichiometry by slc26a3 and slc26a6 is discussed in the context of diseases of epithelial Cl- absorption and HCO3- secretion.
Figures



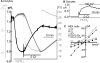
Similar articles
-
Cystic fibrosis transmembrane conductance regulator and SLC26 transporters in HCO₃⁻ secretion by pancreatic duct cells.Sheng Li Xue Bao. 2007 Aug 25;59(4):465-76. Sheng Li Xue Bao. 2007. PMID: 17700966
-
The Slc26a4 transporter functions as an electroneutral Cl-/I-/HCO3- exchanger: role of Slc26a4 and Slc26a6 in I- and HCO3- secretion and in regulation of CFTR in the parotid duct.J Physiol. 2008 Aug 15;586(16):3813-24. doi: 10.1113/jphysiol.2008.154468. Epub 2008 Jun 19. J Physiol. 2008. PMID: 18565999 Free PMC article.
-
Slc26a6: a cardiac chloride-hydroxyl exchanger and predominant chloride-bicarbonate exchanger of the mouse heart.J Physiol. 2004 Dec 15;561(Pt 3):721-34. doi: 10.1113/jphysiol.2004.077339. Epub 2004 Oct 21. J Physiol. 2004. PMID: 15498800 Free PMC article.
-
SLC26 Cl-/HCO3- exchangers in the kidney: roles in health and disease.Kidney Int. 2013 Oct;84(4):657-66. doi: 10.1038/ki.2013.138. Epub 2013 May 1. Kidney Int. 2013. PMID: 23636174 Free PMC article. Review.
-
Apical Cl-/HCO3- exchanger stoichiometry in the modeling of HCO3- transport by pancreatic duct epithelium.J Med Invest. 2009;56 Suppl:325-8. doi: 10.2152/jmi.56.325. J Med Invest. 2009. PMID: 20224216 Review.
Cited by
-
Integration of human and mouse genetics reveals pendrin function in hearing and deafness.Cell Physiol Biochem. 2011;28(3):535-44. doi: 10.1159/000335163. Epub 2011 Nov 18. Cell Physiol Biochem. 2011. PMID: 22116368 Free PMC article. Review.
-
Deletion of Slc26a6 alters the stoichiometry of apical Cl-/HCO-3 exchange in mouse pancreatic duct.Am J Physiol Cell Physiol. 2012 Oct 15;303(8):C815-24. doi: 10.1152/ajpcell.00151.2012. Epub 2012 Aug 15. Am J Physiol Cell Physiol. 2012. PMID: 22895259 Free PMC article.
-
CFTR functions as a bicarbonate channel in pancreatic duct cells.J Gen Physiol. 2009 Mar;133(3):315-26. doi: 10.1085/jgp.200810122. Epub 2009 Feb 9. J Gen Physiol. 2009. PMID: 19204187 Free PMC article.
-
Congenital chloride-losing diarrhea causing mutations in the STAS domain result in misfolding and mistrafficking of SLC26A3.J Biol Chem. 2008 Mar 28;283(13):8711-22. doi: 10.1074/jbc.M704328200. Epub 2008 Jan 23. J Biol Chem. 2008. PMID: 18216024 Free PMC article.
-
Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells.J Biol Chem. 2011 Nov 25;286(47):41069-82. doi: 10.1074/jbc.M111.266734. Epub 2011 Sep 13. J Biol Chem. 2011. PMID: 21914796 Free PMC article.
References
-
- Accardi, A., and C. Miller. 2004. Secondary active transport mediated by a prokaryotic homologue of ClC Cl- channels. Nature. 427:803–807. - PubMed
-
- Bissig, M., B. Hagenbuch, B. Stieger, T. Koller, and P.J. Meier. 1994. Functional expression cloning of the canalicular sulfate transport system of rat hepatocytes. J. Biol. Chem. 269:3017–3021. - PubMed
-
- Boron, W.F. 1986. Intracellular pH regulation in epithelial cells. Annu. Rev. Physiol. 48:377–388. - PubMed
-
- Chernova, M.N., L. Jiang, D.J. Friedman, R.B. Darman, H. Lohi, J. Kere, D.H. Vandorpe, and S.L. Alper. 2005. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J. Biol. Chem. 280:8564–8580. - PubMed
-
- Cook, D.I., E.W. Van Lennep, M.L. Roberts, and J.A. Young. 1994. Secretion by the major salivary glands. In Textbook of Physiology of the Gastrointestinal Tract. L.R. Johnson, editor. Raven Press, New York. 1061–1117.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

