A dynamic ubiquitin equilibrium couples proteasomal activity to chromatin remodeling
- PMID: 16606690
- PMCID: PMC2063781
- DOI: 10.1083/jcb.200510071
A dynamic ubiquitin equilibrium couples proteasomal activity to chromatin remodeling
Abstract
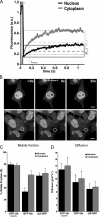
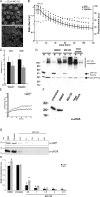
Protein degradation, chromatin remodeling, and membrane trafficking are critically regulated by ubiquitylation. The presence of several coexisting ubiquitin-dependent processes, each of crucial importance to the cell, is remarkable. This brings up questions on how the usage of this versatile regulator is negotiated between the different cellular processes. During proteotoxic stress, the accumulation of ubiquitylated substrates coincides with the depletion of ubiquitylated histone H2A and chromatin remodeling. We show that this redistribution of ubiquitin during proteotoxic stress is a direct consequence of competition for the limited pool of free ubiquitin. Thus, the ubiquitin cycle couples various ubiquitin-dependent processes because of a rate-limiting pool of free ubiquitin. We propose that this ubiquitin equilibrium may allow cells to sense proteotoxic stress in a genome-wide fashion.
Figures



References
-
- Aguilar, R.C., and B. Wendland. 2003. Ubiquitin: not just for proteasomes anymore. Curr. Opin. Cell Biol. 15:184–190. - PubMed
-
- de Napoles, M., J.E. Mermoud, R. Wakao, Y.A. Tang, M. Endoh, R. Appanah, T.B. Nesterova, J. Silva, A.P. Otte, M. Vidal, et al. 2004. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev. Cell. 7:663–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

