Lamina-associated polypeptide 2alpha regulates cell cycle progression and differentiation via the retinoblastoma-E2F pathway
- PMID: 16606692
- PMCID: PMC2063793
- DOI: 10.1083/jcb.200511149
Lamina-associated polypeptide 2alpha regulates cell cycle progression and differentiation via the retinoblastoma-E2F pathway
Abstract
Lamina-associated polypeptide (LAP) 2alpha is a nonmembrane-bound LAP2 isoform that forms complexes with nucleoplasmic A-type lamins. In this study, we show that the overexpression of LAP2alpha in fibroblasts reduced proliferation and delayed entry into the cell cycle from a G0 arrest. In contrast, stable down-regulation of LAP2alpha by RNA interference accelerated proliferation and interfered with cell cycle exit upon serum starvation. The LAP2alpha-linked cell cycle phenotype is mediated by the retinoblastoma (Rb) protein because the LAP2alpha COOH terminus directly bound Rb, and overexpressed LAP2alpha inhibited E2F/Rb-dependent reporter gene activity in G1 phase in an Rb-dependent manner. Furthermore, LAP2alpha associated with promoter sequences in endogenous E2F/Rb-dependent target genes in vivo and negatively affected their expression. In addition, the expression of LAP2alpha in proliferating preadipocytes caused the accumulation of hypophosphorylated Rb, which is reminiscent of noncycling cells, and initiated partial differentiation into adipocytes. The effects of LAP2alpha on cell cycle progression and differentiation may be highly relevant for the cell- and tissue-specific phenotypes observed in laminopathic diseases.
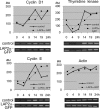
Figures





References
-
- Arimura, T., A. Helbling-Leclerc, C. Massart, S. Varnous, F. Niel, E. Lacene, Y. Fromes, M. Toussaint, A.M. Mura, D.I. Keller, et al. 2005. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet. 14:155–169. - PubMed
-
- Berger, R., L. Theodor, J. Shoham, E. Gokkel, F. Brok-Simoni, K.B. Avraham, N.G. Copeland, N.A. Jenkins, G. Rechavi, and A.J. Simon. 1996. The characterization and localization of the mouse thymopoietin/lamina-associated polypeptide 2 gene and its alternatively spliced products. Genome Res. 6:361–370. - PubMed
-
- Boguslavsky, R.L., C.L. Stewart, and H.J. Worman. 2006. Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type familial partial lipodystrophy. Hum. Mol. Genet. 15:653–663. - PubMed
-
- Brehm, A., E.A. Miska, D.J. McCance, J.L. Reid, A.J. Bannister, and T. Kouzarides. 1998. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 391:597–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

