Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo
- PMID: 16606824
- PMCID: PMC1458875
- DOI: 10.1073/pnas.0601755103
Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo
Abstract
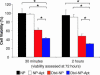
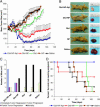
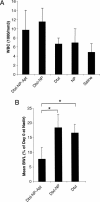
Targeted uptake of therapeutic nanoparticles in a cell-, tissue-, or disease-specific manner represents a potentially powerful technology. Using prostate cancer as a model, we report docetaxel (Dtxl)-encapsulated nanoparticles formulated with biocompatible and biodegradable poly(D,L-lactic-co-glycolic acid)-block-poly(ethylene glycol) (PLGA-b-PEG) copolymer and surface functionalized with the A10 2'-fluoropyrimidine RNA aptamers that recognize the extracellular domain of the prostate-specific membrane antigen (PSMA), a well characterized antigen expressed on the surface of prostate cancer cells. These Dtxl-encapsulated nanoparticle-aptamer bioconjugates (Dtxl-NP-Apt) bind to the PSMA protein expressed on the surface of LNCaP prostate epithelial cells and get taken up by these cells resulting in significantly enhanced in vitro cellular toxicity as compared with nontargeted nanoparticles that lack the PSMA aptamer (Dtxl-NP) (P < 0.0004). The Dtxl-NP-Apt bioconjugates also exhibit remarkable efficacy and reduced toxicity as measured by mean body weight loss (BWL) in vivo [body weight loss of 7.7 +/- 4% vs. 18 +/- 5% for Dtxl-NP-Apt vs. Dtxl-NP at nadir, respectively (mean +/- SD); n = 7]. After a single intratumoral injection of Dtxl-NP-Apt bioconjugates, complete tumor reduction was observed in five of seven LNCaP xenograft nude mice (initial tumor volume of approximately 300 mm3), and 100% of these animals survived our 109-day study. In contrast, two of seven mice in the Dtxl-NP group had complete tumor reduction with 109-day survivability of only 57%. Dtxl alone had a survivability of only 14%. Saline and nanoparticles without drug were similarly nonefficacious. This report demonstrates the potential utility of nanoparticle-aptamer bioconjugates for a therapeutic application.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
-
- Grimm P. D., Blasko J. C., Sylvester J. E., Meier R. M., Cavanagh W. Int. J. Radiat. Oncol. Biol. Phys. 2001;51:31–40. - PubMed
-
- Cooperberg M. R., Moul J. W., Carroll P. R. J. Clin. Oncol. 2005;23:8146–8151. - PubMed
-
- Teloken C. Cancer Control. 2001;8:540–545. - PubMed
-
- Han B. H., Demel K. C., Wallner K., Ellis W., Young L., Russell K. J. Urol. 2001;166:953–957. - PubMed
-
- Bucci J., Morris W. J., Keyes M., Spadinger I., Sidhu S., Moravan V. Int. J. Radiat. Oncol. Biol. Phys. 2002;53:91–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

