The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli
- PMID: 16606827
- PMCID: PMC1458889
- DOI: 10.1073/pnas.0601620103
The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli
Abstract
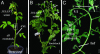
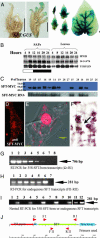
The systemic model for floral induction, dubbed florigen, was conceived in photoperiod-sensitive plants but implies, in its ultimate form, a graft-transmissible signal that, although activated by different stimuli in different flowering systems, is common to all plants. We show that SFT (SINGLE-FLOWER TRUSS), the tomato ortholog of FLOWERING LOCUS T (FT), induces flowering in day-neutral tomato and tobacco plants and is encoded by SFT. sft tomato mutant plants are late-flowering, with altered architecture and flower morphology. SFT-dependent graft-transmissible signals complement all developmental defects in sft plants and substitute for long-day stimuli in Arabidopsis, short-day stimuli in Maryland Mammoth tobacco, and light-dose requirements in tomato uniflora mutant plants. The absence of donor SFT RNA from flowering receptor shoots and the localization of the protein in leaf nuclei implicate florigen-like messages in tomato as a downstream pathway triggered by cell-autonomous SFT RNA transcripts. Flowering in tomato is synonymous with termination of the shoot apical meristems, and systemic SFT messages attenuate the growth of apical meristems before and independent of floral production. Floral enhancement by systemic SFT signals is therefore one pleiotropic effect of FT orthologs.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
-
- Chailakhyan M. K. Dokl. Akad. Nauk SSSR. 1936;13:79–83.
-
- Zeevaart J. A. D. Annu. Rev. Plant Physiol. 1976;27:321–348.
-
- Vince-Prue D. Photoperiodism in Plants. London: McGraw–Hill; 1975.
-
- Blazquez M. A., Weigel D. Nature. 2000;404:889–892. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

