Bone marrow-derived cells fuse with normal and transformed intestinal stem cells
- PMID: 16606845
- PMCID: PMC1435365
- DOI: 10.1073/pnas.0508593103
Bone marrow-derived cells fuse with normal and transformed intestinal stem cells
Abstract
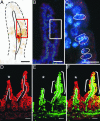
Transplanted adult bone marrow-derived cells (BMDCs) have been shown to adopt the phenotype and function of several nonhematopoietic cell lineages and promote tumorigenesis. Beyond its cancer enhancing potential, cell fusion has recently emerged as an explanation of how BMDCs regenerate diseased heptocytes, contribute to Purkinje neurons and skeletal and cardiac muscle cells, and participate in skin and heart regeneration. Although bone marrow-derived epithelial cells also have been observed in the intestine, fusion as a mechanism has not been investigated. Here, we show that transplanted BMDCs fuse with both normal and neoplastic intestinal epithelium. Long-term repopulation by donor-derived cells was detected in all principal intestinal epithelial lineages including enterocytes, goblet cells, Paneth cells, and enteroendocrine cells, suggesting that the fusion partners of the BMDCs are long-lived intestinal progenitors or stem cells. Fusion of BMDCs with neoplastic epithelium did not result in tumor initiation. Our findings suggest an unexpected role for BMDCs in both regeneration and tumorigenesis of the intestine.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
-
- Lagasse E., Connors H., Al-Dhalimy M., Reitsma M., Dohse M., Osborne L., Wang X., Finegold M., Weissman I. L., Grompe M. Nat. Med. 2000;6:1229–1234. - PubMed
-
- Rasulov M. F., Vasilchenkov A. V., Onishchenko N. A., Krasheninnikov M. E., Kravchenko V. I., Gorshenin T. L., Pidtsan R. E., Potapov I. V. Bull. Exp. Biol. Med. 2005;139:141–144. - PubMed
-
- Alvarez-Dolado M., Pardal R., Garcia-Verdugo J. M., Fike J. R., Lee H. O., Pfeffer K., Lois C., Morrison S. J., Alvarez-Buylla A. Nature. 2003;425:968–973. - PubMed
-
- Laflamme M. A., Myerson D., Saffitz J. E., Murry C. E. Circ. Res. 2002;90:634–640. - PubMed
-
- Nygren J. M., Jovinge S., Breitbach M., Sawen P., Roll W., Hescheler J., Taneera J., Fleischmann B. K., Jacobsen S. E. Nat. Med. 2004;10:494–501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

